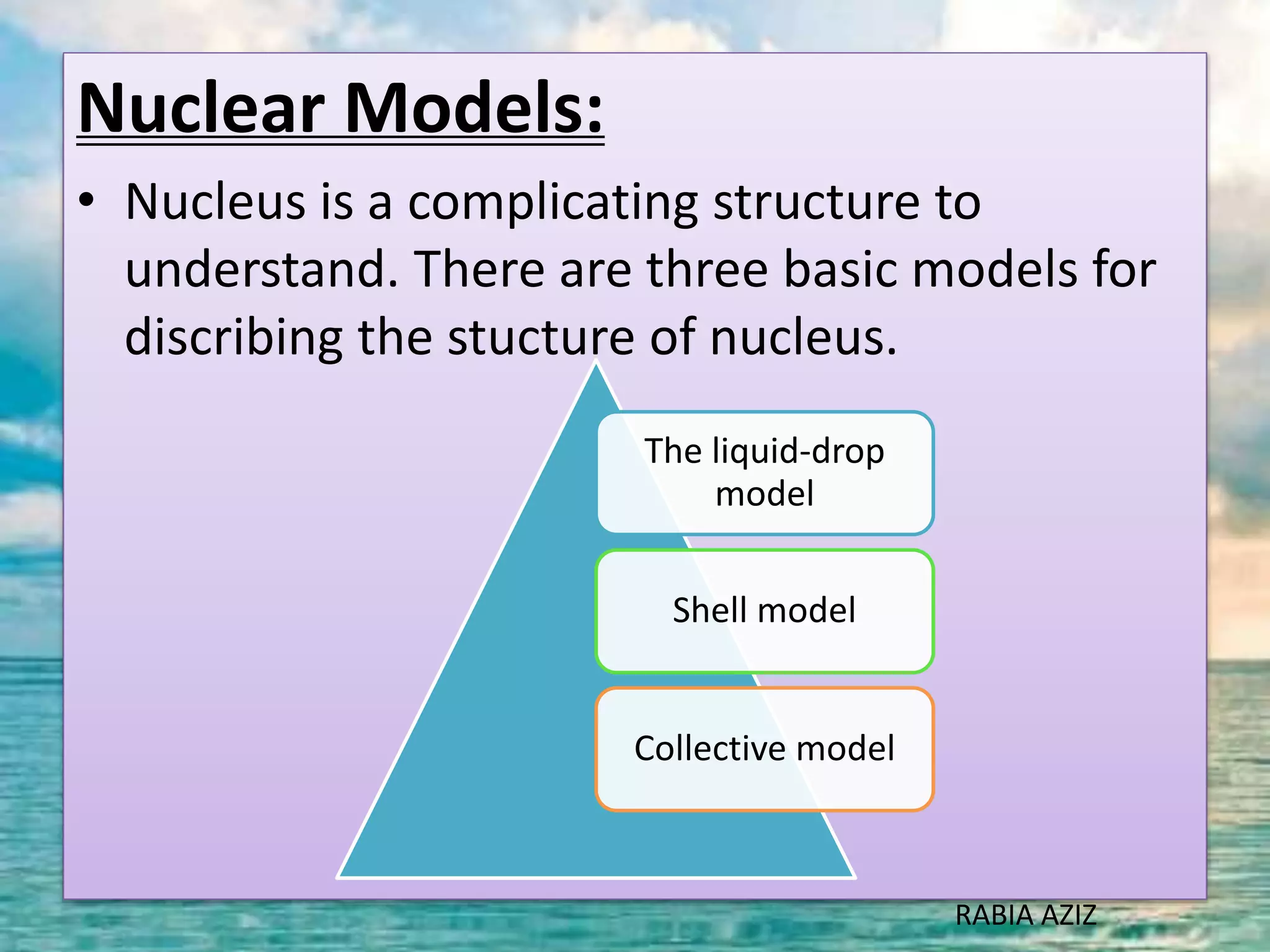
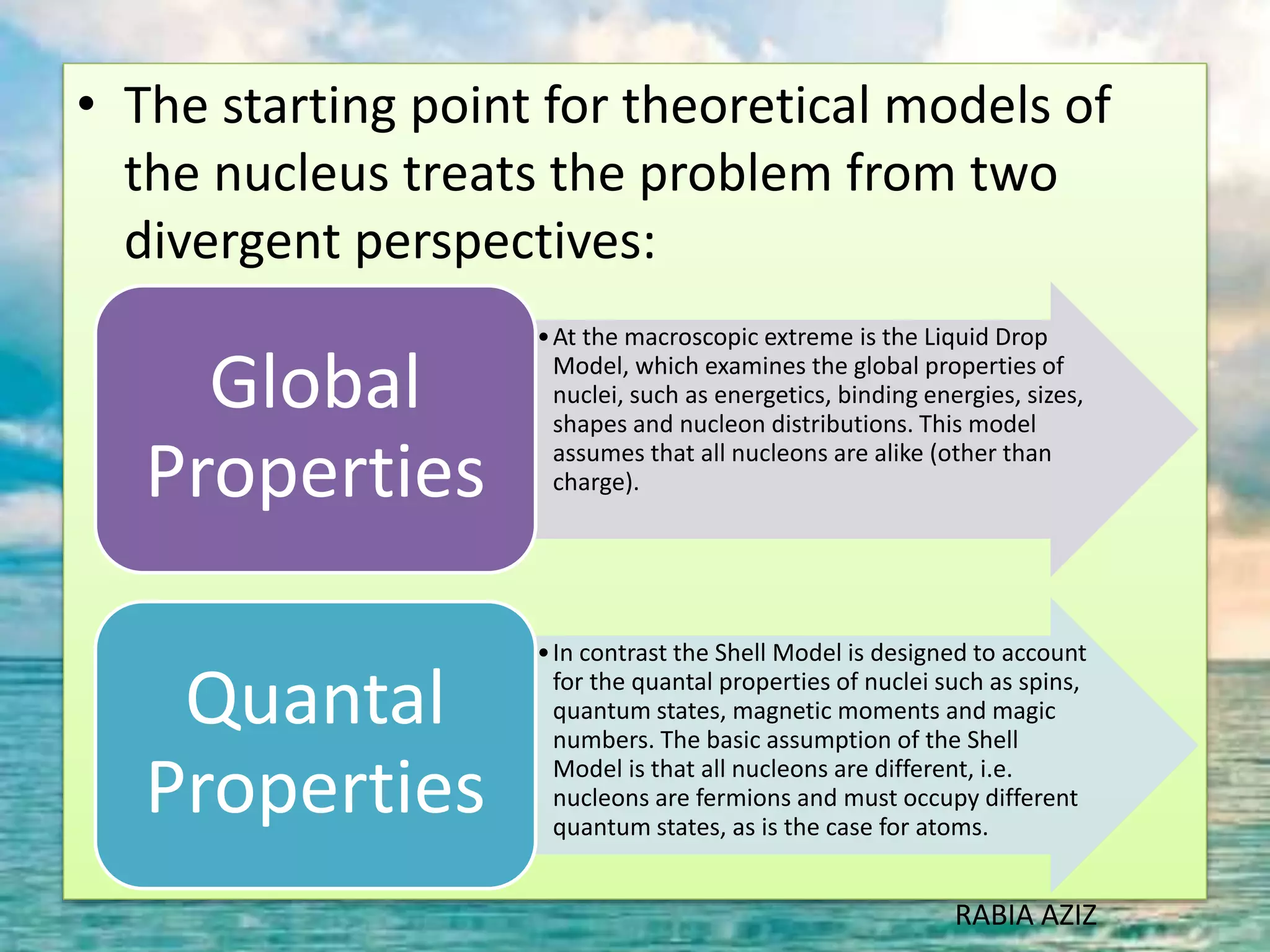
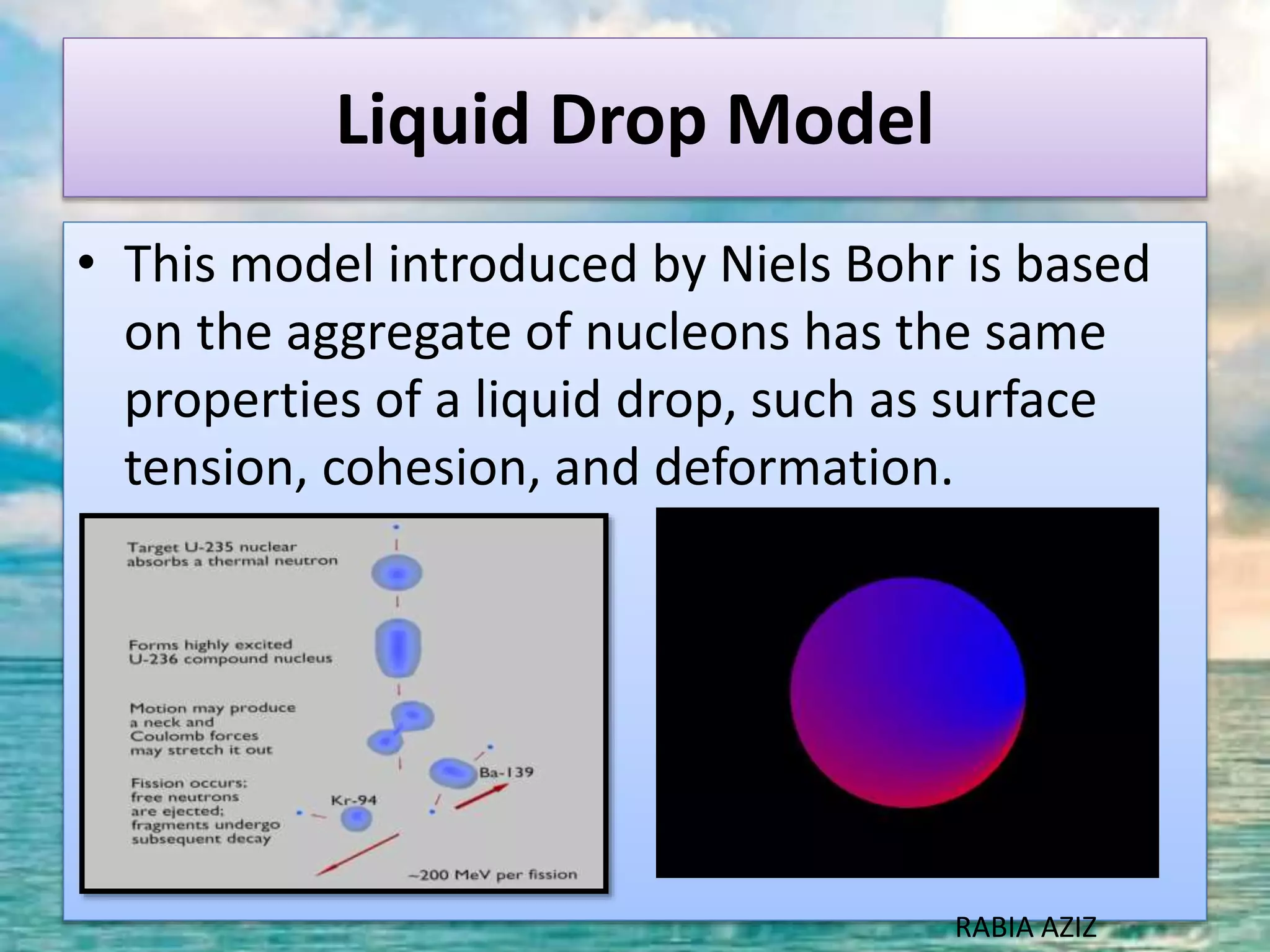
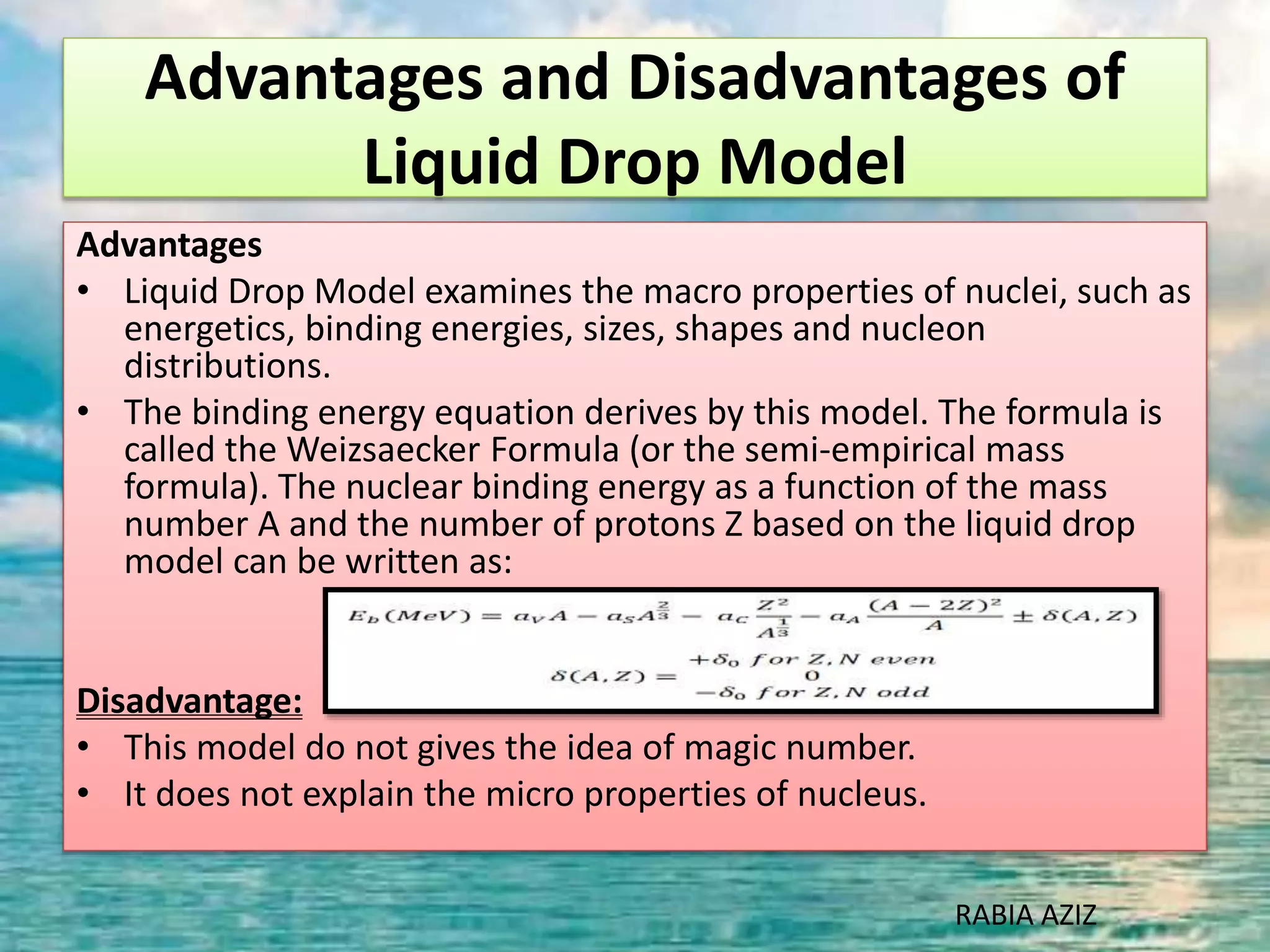
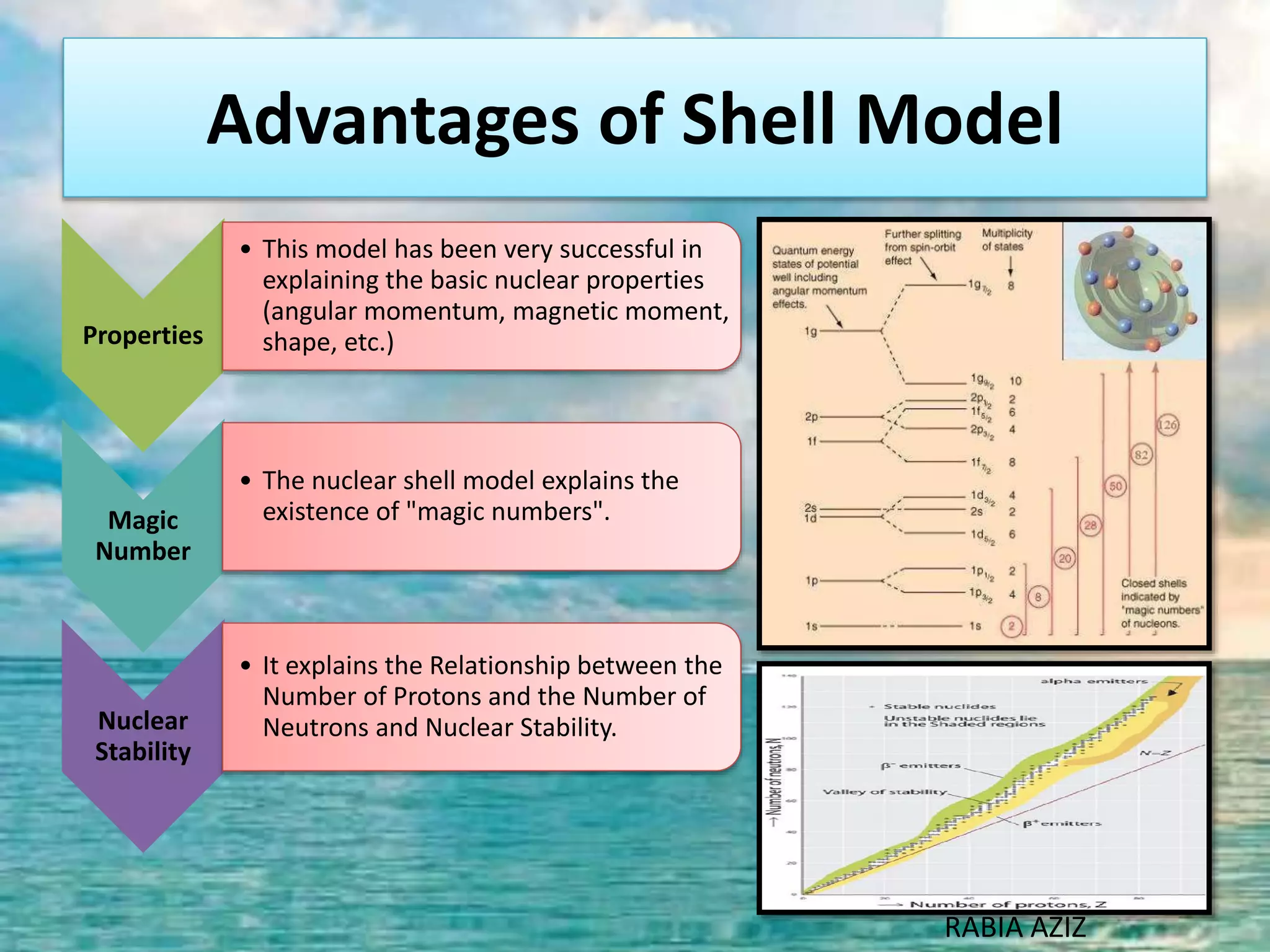
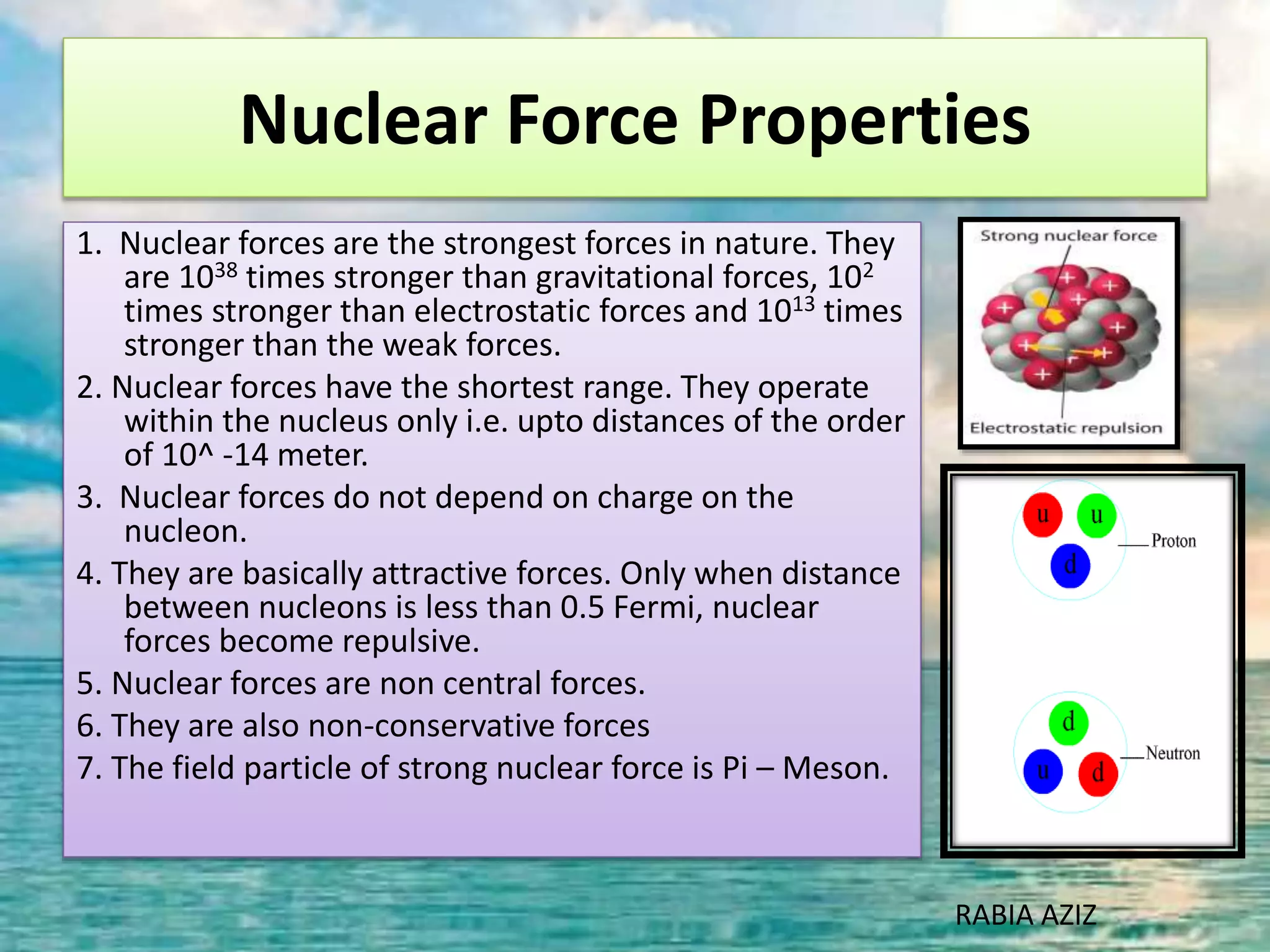
The document discusses nuclear models and nuclear forces. It describes three nuclear models: the liquid drop model, shell model, and collective model. The liquid drop model treats the nucleus as a liquid drop and examines its global properties. The shell model arranges nucleons into energy shells like electrons in an atom and explains magic numbers. The collective model incorporates aspects of the liquid drop and shell models. Nuclear forces operate very strongly within the nucleus, are attractive between protons and neutrons, and are responsible for nuclear stability.