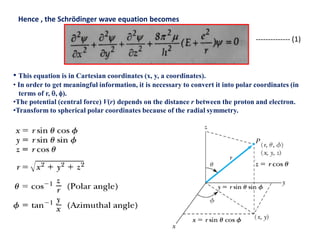
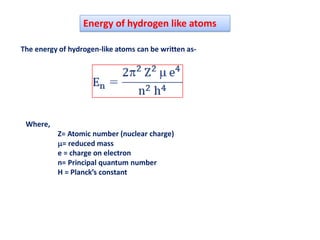
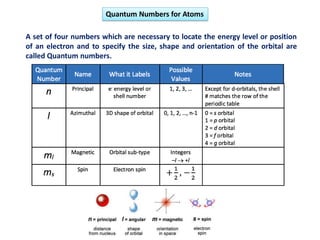
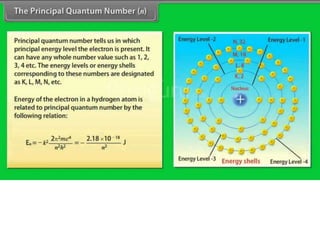
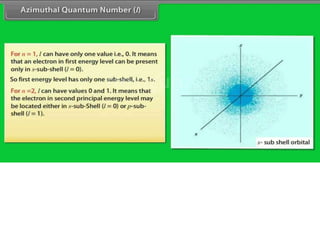
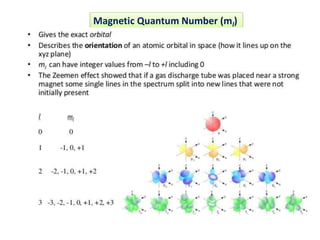
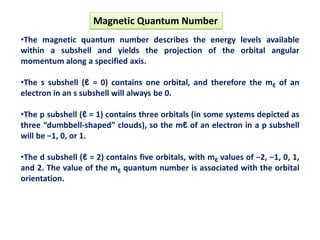
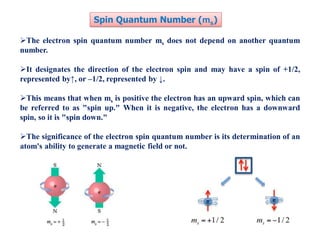
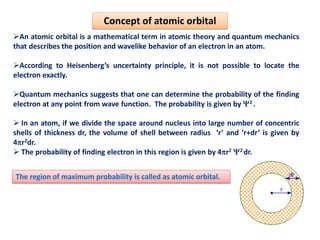
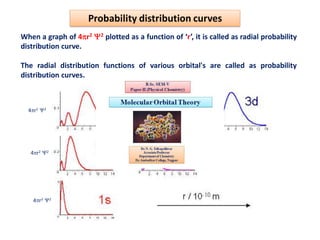
This document discusses the Schrodinger wave equation for hydrogen atoms. It begins by presenting the time-independent 3D Schrodinger wave equation and explains how it is converted to polar coordinates due to the radial symmetry of hydrogen atoms. The wave function is assumed to separate into three parts, leading to three equations involving the principal, azimuthal, and magnetic quantum numbers. Quantum numbers and their relationships to orbital shapes are also described. Finally, atomic orbitals are defined as regions of high probability of finding electrons based on the Schrodinger wave equation solution.