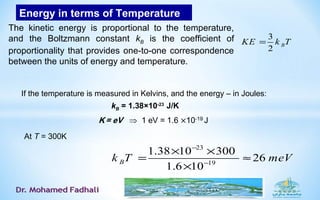
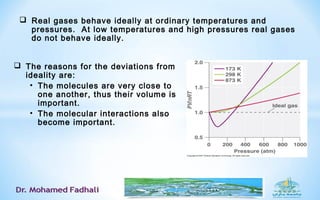
Ideal gases approximate the behavior of real gases at low pressures and densities. The kinetic theory of gases describes ideal gases as large numbers of tiny particles that move freely and undergo elastic collisions. The kinetic theory assumptions lead to simple relationships between pressure, volume, temperature, and number of moles or particles for ideal gases. The van der Waals equation accounts for the finite size of gas particles and their intermolecular attractions, better describing the behavior of real gases that deviate from ideal gas behavior at high pressures and low temperatures.

![The Equation of State of Ideal Gases
P – pressure [N/m2
]
V – volume [m3
]
n – number of moles of gas
T – the temperature in Kelvins [K]
R – a universal constant
nRTPV =
Kmol
J
R
⋅
≈ 31.8
The ideal gas
equation of state:
An equation that relates macroscopic variables (e.g., P, V, and T) for a given
substance in thermodynamic equilibrium.
In equilibrium (≡ no macroscopic motion), just a few macroscopic parameters are
required to describe the state of a system.
f (P,V,T) = 0
Geometrical
representation of the
equation of state:
P
V
T
an equilibrium
state
the equation-
of-state surface
R= 0.08214 L atm/mol K](https://image.slidesharecdn.com/thermodynamicpart-2-160902101809/85/Thermodynamic-part-2-6-320.jpg)
![Mm – Molar mass [g/mol]
N – Number of molecules [molecules]
NA – Avogadro’s Number of molecules per mole
[molecules/mol]
mM
m
n =
AN
N
n =
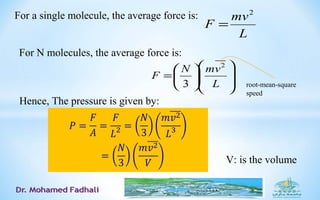
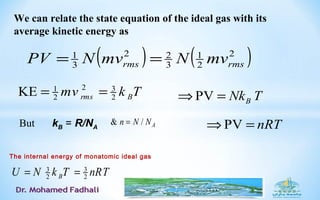
Where,TNkPV B=
the Boltzmann constant
kB = R/NA ≈ 1.38⋅10-23
J/K
(introduced by Planck in 1899)
Avogadro’s Law: equal volumes of different gases at the same P
and T contain the same amount of molecules.
Ideal gas, constant mass (fixed quantity of gas)
1 1 2 2
1 2
PV P V
T T
=](https://image.slidesharecdn.com/thermodynamicpart-2-160902101809/85/Thermodynamic-part-2-7-320.jpg)



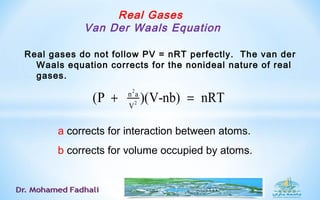

![Real Gases :Van Der Waals Equation
The attractive forces between real molecules, which reduce the pressure:
p ∝ wall collision frequency and
p ∝ change in momentum at each collision.
Both factors are proportional to concentration, n/V, and p is reduced by
an amount a(n/V)2
, where a depends on the type of gas.
[Note: a/V2
is called the internal pressure of the gas].
2
2
n a
V
P becomes (P )+
n2
a/V2
represents the effect on pressure to
intermolecular attractions or repulsions.](https://image.slidesharecdn.com/thermodynamicpart-2-160902101809/85/Thermodynamic-part-2-17-320.jpg)