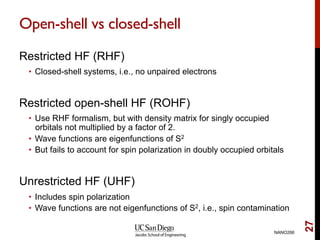
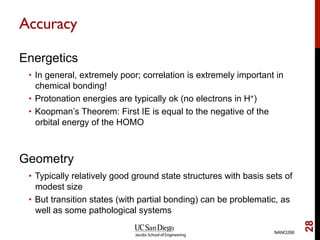
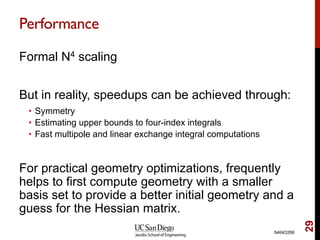
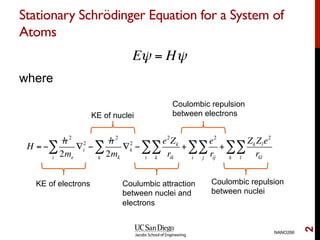
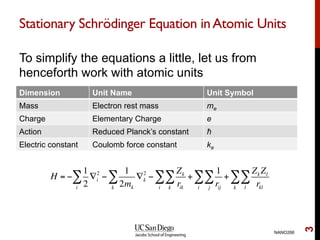
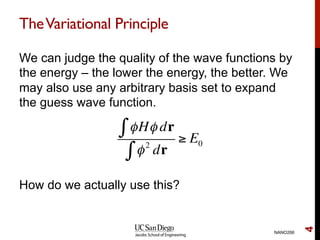
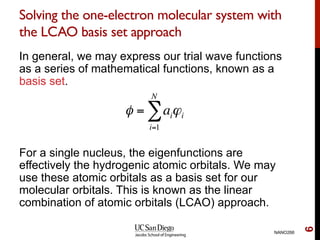
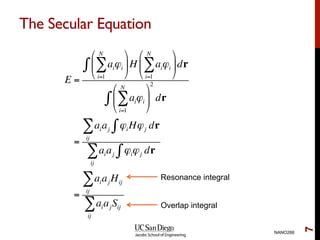
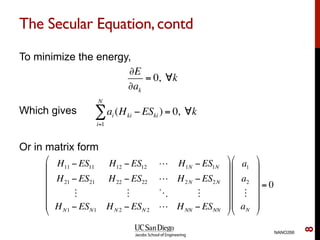
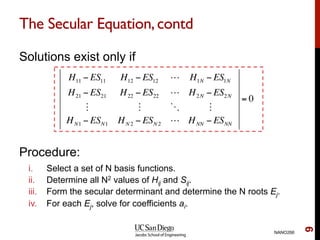
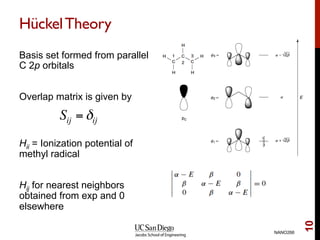
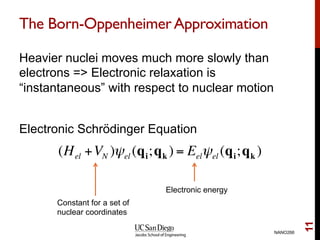
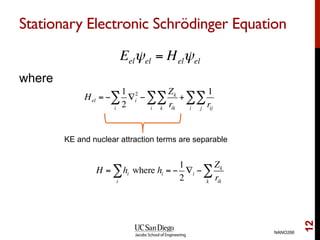
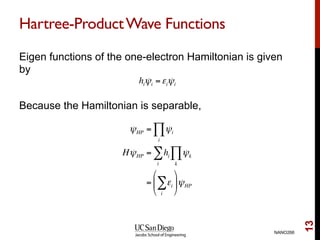
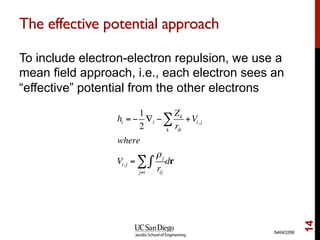
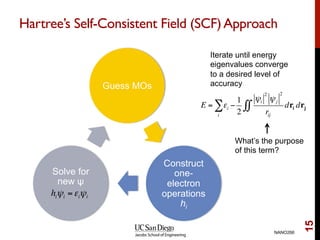
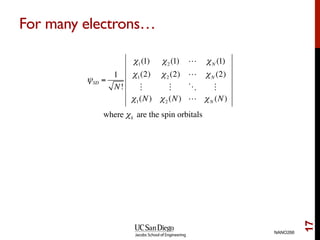
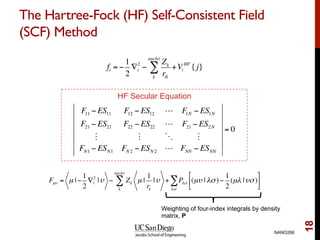
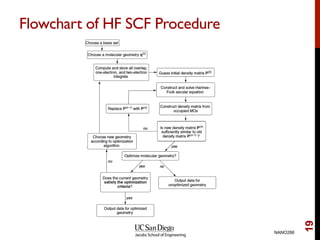
The document discusses the Hartree-Fock approximation and the stationary Schrödinger equation for atomic systems, emphasizing the significance of the variational principle in determining the quality of wave functions based on energy levels. It elaborates on the Linear Combination of Atomic Orbitals (LCAO) approach and the secular equation used to minimize energy in one-electron molecular systems. Additionally, it covers limitations of the Hartree-Fock method, basis sets, and performance considerations, including effective core potentials and strategies to improve computational efficiency.

![What about the Pauli Exclusion Principle?
Two identical fermions (spin ½ particles) cannot
occupy the same quantum state simultaneously
è Wave function has to be anti-symmetric
For two electron system, we have
NANO266
16
ψSD =
1
2
ψa (1)α(1)ψb (2)α(2)−ψa (2)α(2)ψb (1)α(1)[ ]
=
1
2
ψa (1)α(1) ψb (1)α(1)
ψa (2)α(2) ψb (2)α(2)
where α is the electron spin eigenfunction
Slater determinant](https://image.slidesharecdn.com/02-thehartree-fockapproach-150406231642-conversion-gate01/85/NANO266-Lecture-2-The-Hartree-Fock-Approach-16-320.jpg)

![Polarization and Diffuse Functions
Polarization functions
• Description of MOs require more flexibility than provided by AOs,
e.g., NH3 is predicted to be planar if using just s and p functions
• Additional basis functions of one quantum number of higher
angular momentum than valence, e.g., first row -> d orbitals
• Notation: 6-31G* [old] or 6-31G(d) [new], 6-31(2d,p) [2d functions
for heavy atoms, additional p for H]
Diffuse functions
• Highest energy MOs of anions, highly excited states tend to be
more diffuse
• Augment standard basis sets with diffuse functions
• Notation: 6-31+G, 6-311++G(3df, 2pd), aug-cc-pCVDZ
NANO266
25](https://image.slidesharecdn.com/02-thehartree-fockapproach-150406231642-conversion-gate01/85/NANO266-Lecture-2-The-Hartree-Fock-Approach-25-320.jpg)