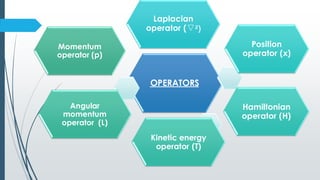
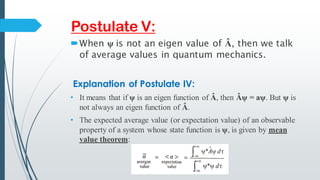
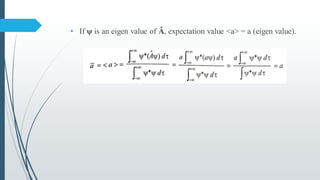
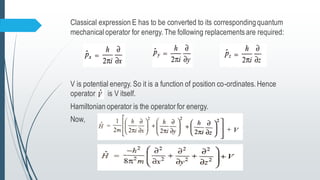
The document outlines fundamental postulates of quantum mechanics, beginning with the significance of the wave function (ψ) as a key property describing a system's state. It details properties of ψ, conditions for eigenfunctions, and the relationship between classical observables and quantum mechanical operators, including the process of obtaining eigenvalues. Additionally, it discusses the derivation of the time-independent Schrödinger wave equation using these postulates to connect classical energy expressions to quantum mechanics.