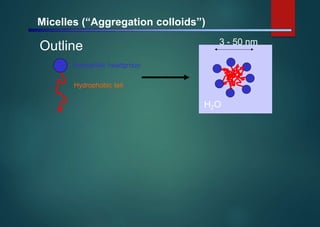
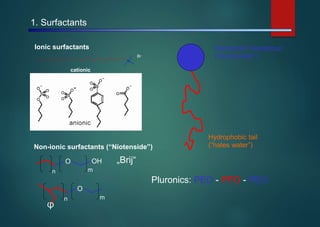
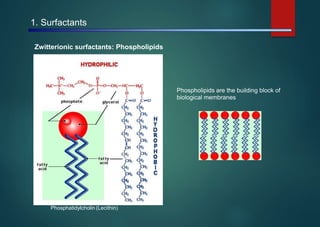
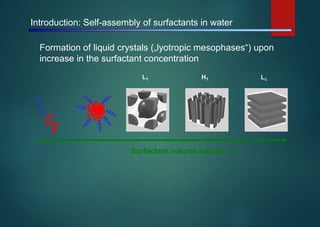
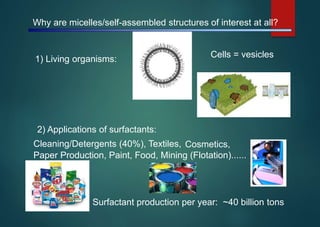
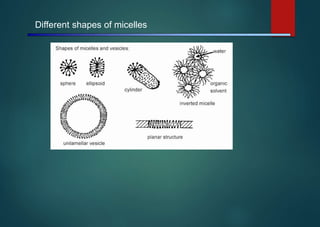
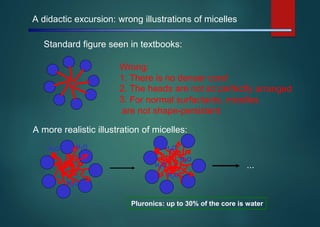
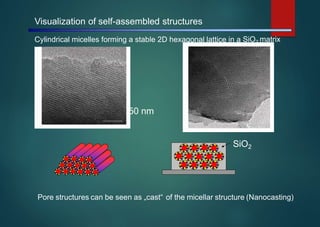
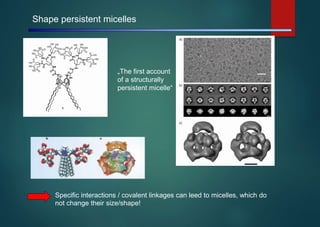
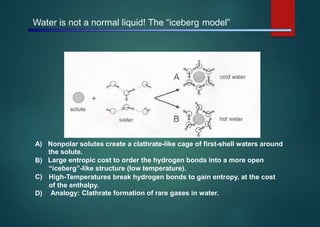
- Micelles are aggregates of surfactant molecules that form spherical structures in water, with the hydrophilic "heads" facing outwards and the hydrophobic tails sequestered in the inner core.
- They are typically 3-50 nm in size and form as a result of the packing behavior of single-tailed lipids, which leads to aggregation that allows accommodation of the head groups at the interface while filling the interior volume.
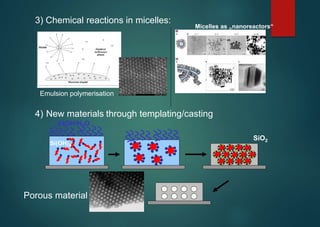
- Micelles have various applications including in detergents, cosmetics, and as nanoreactors for chemical reactions, and their formation is driven entropically by the ordering of water molecules despite the enthalpic cost of aggregation.