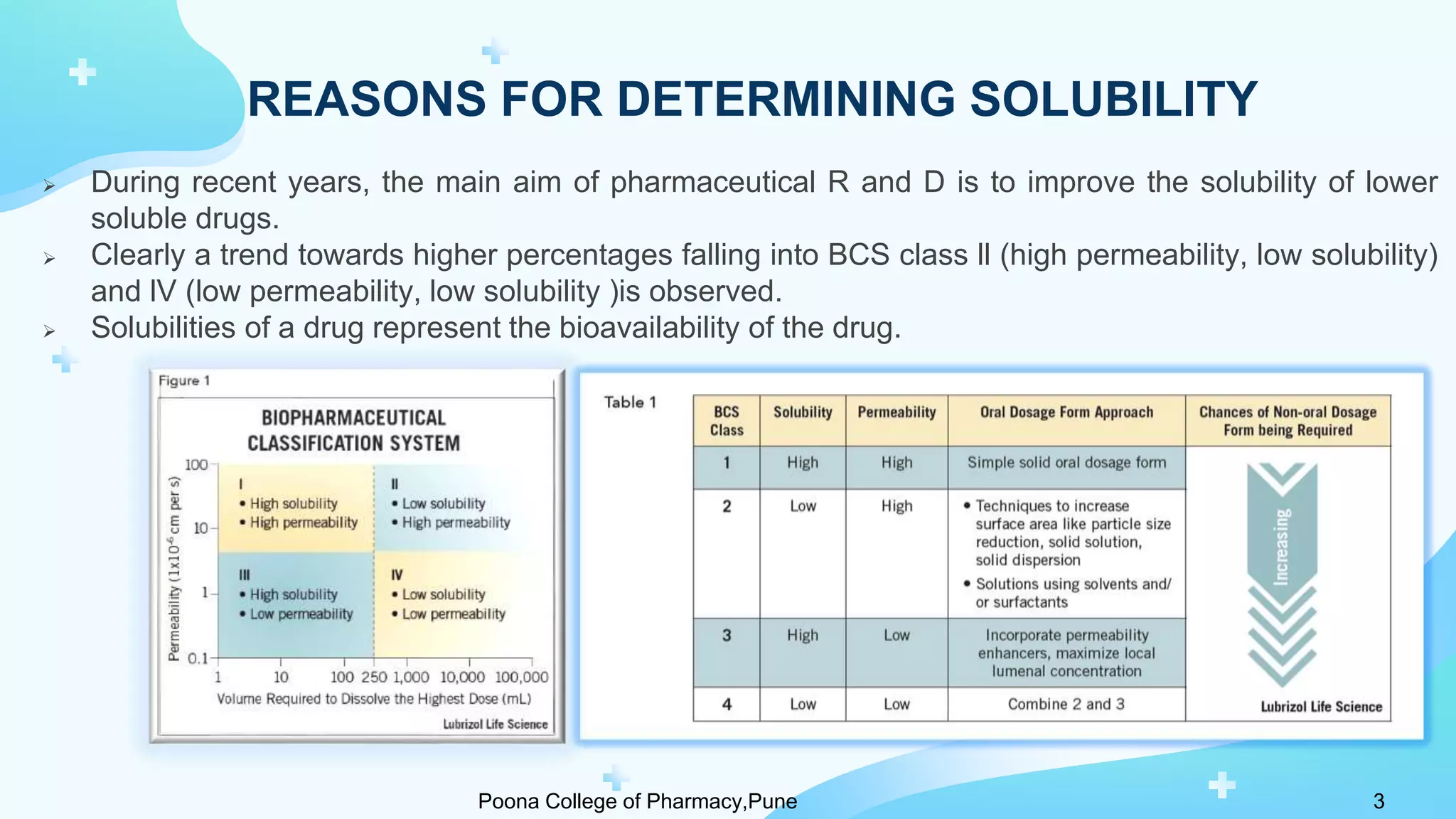
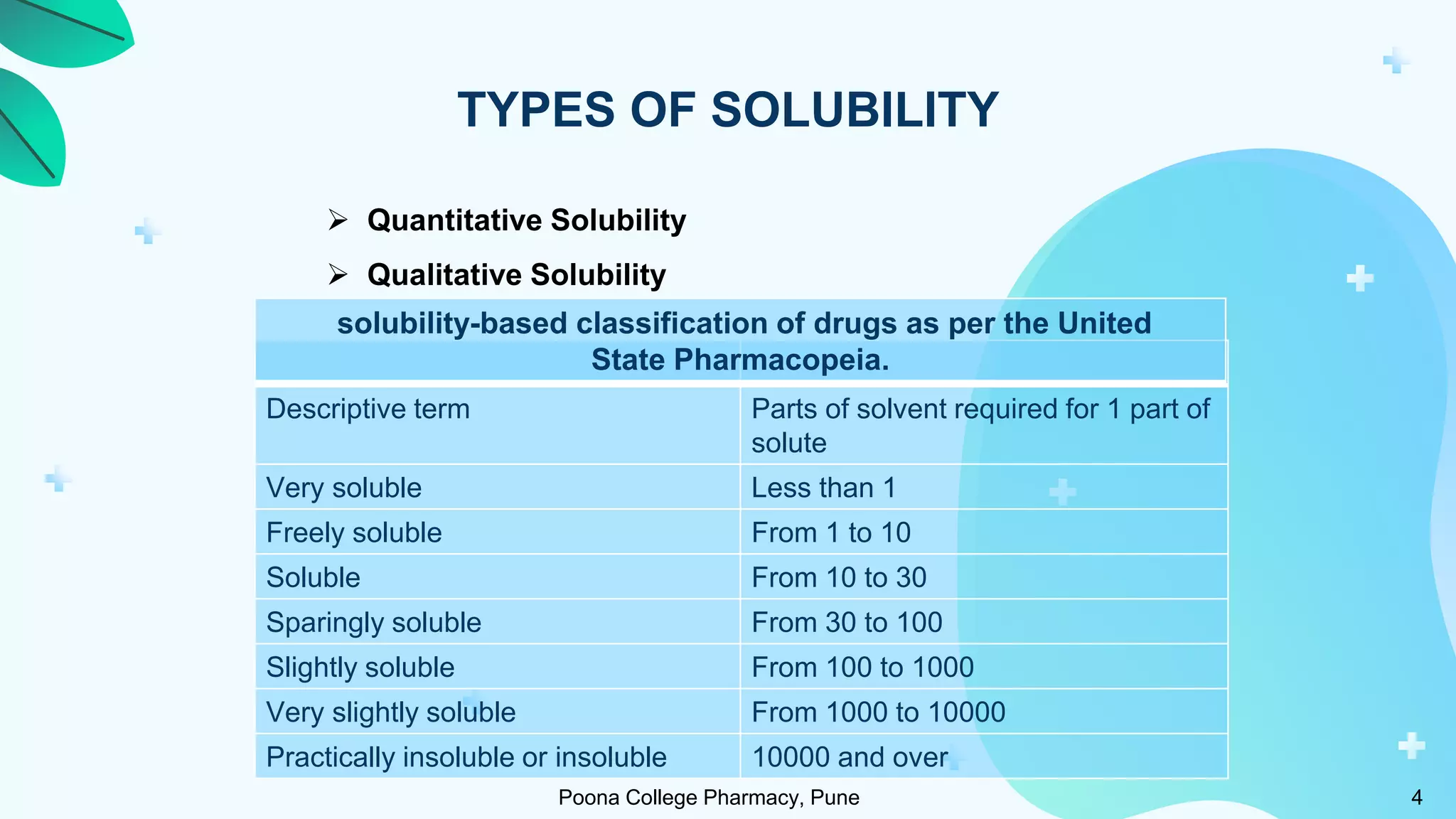
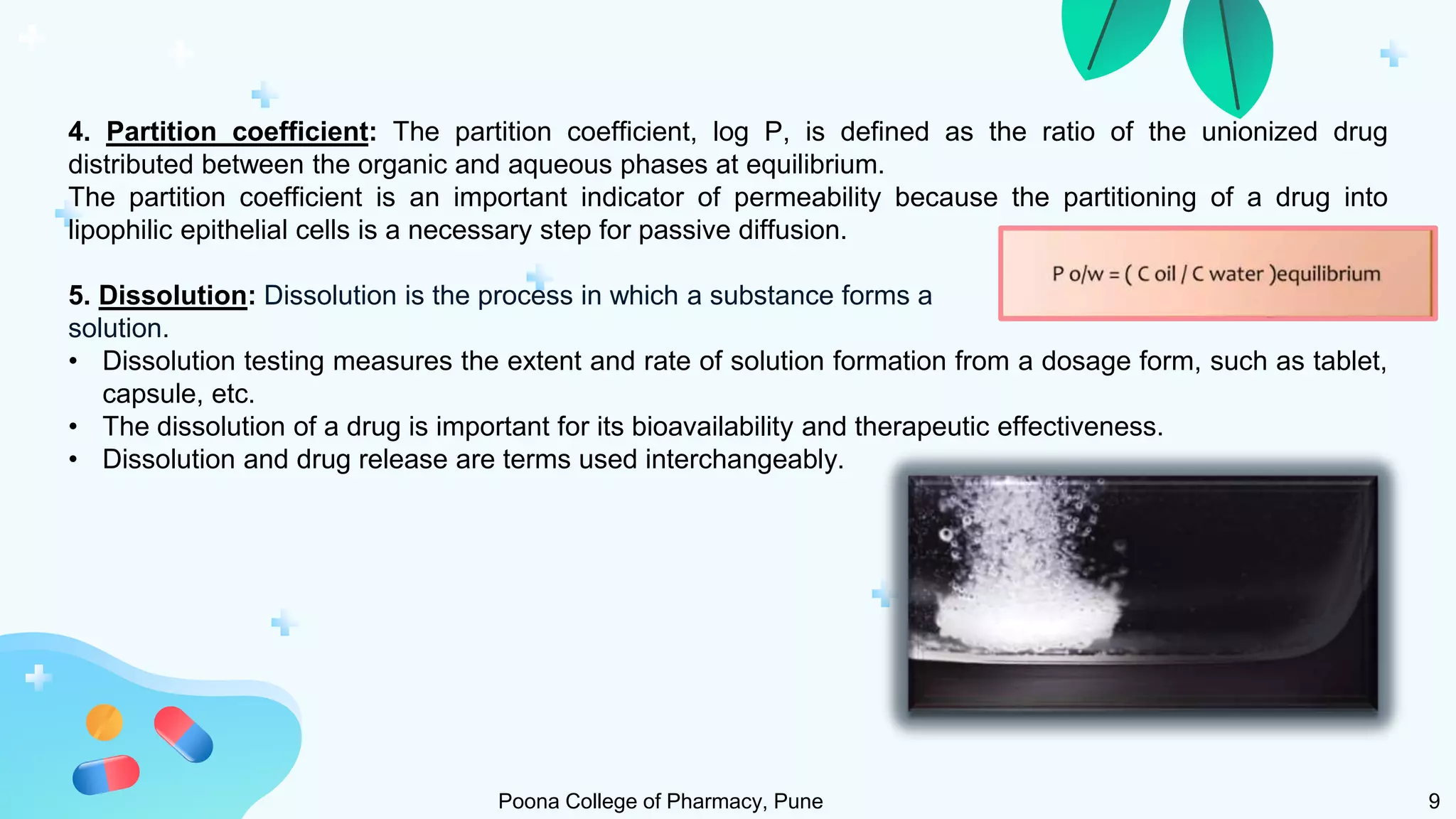
1) Solubility refers to the maximum amount of a substance that dissolves in a solvent to make a saturated solution at a given temperature and pressure. Solubility is important for drug bioavailability and is measured at 4°C and 37°C.
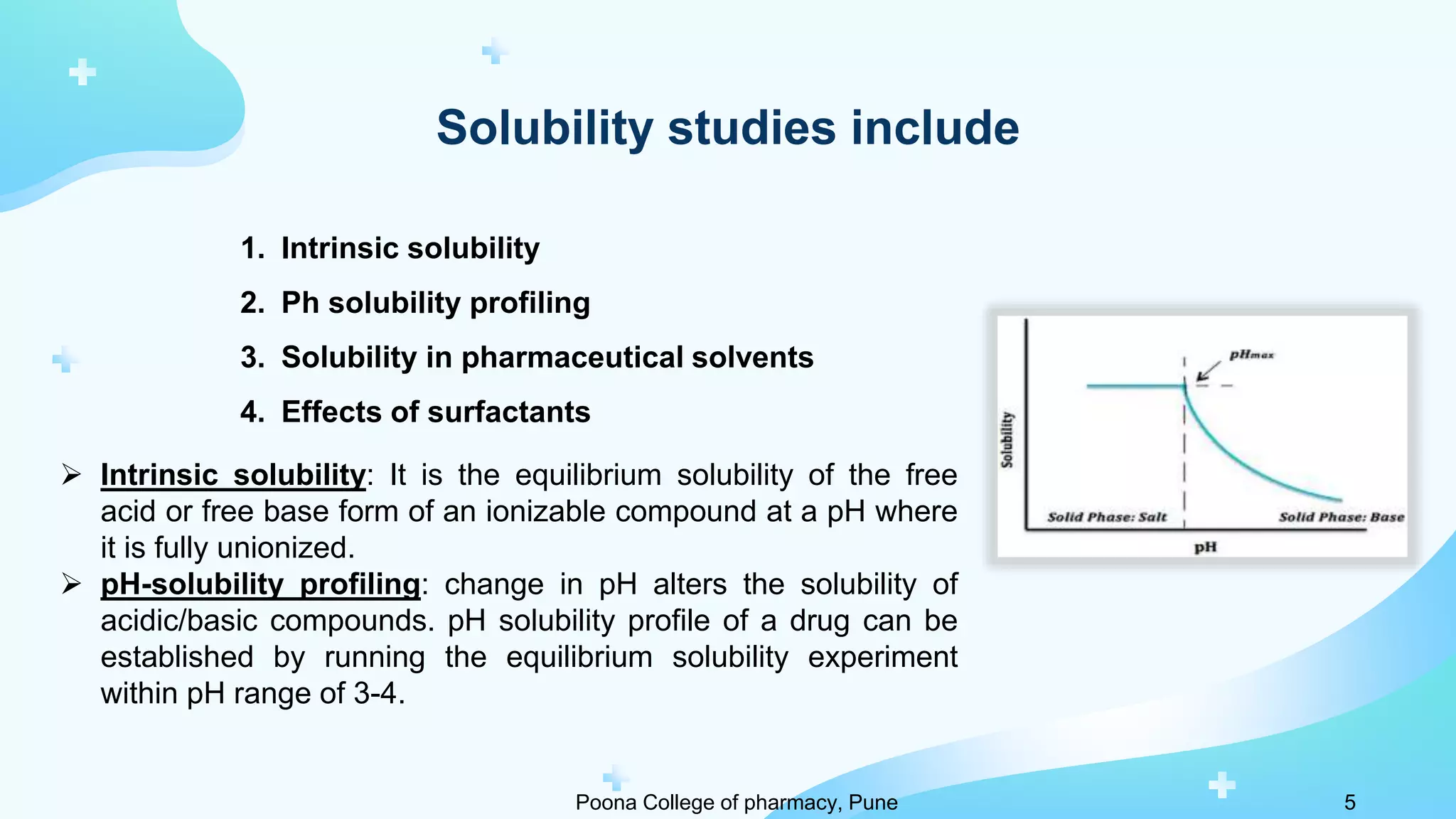
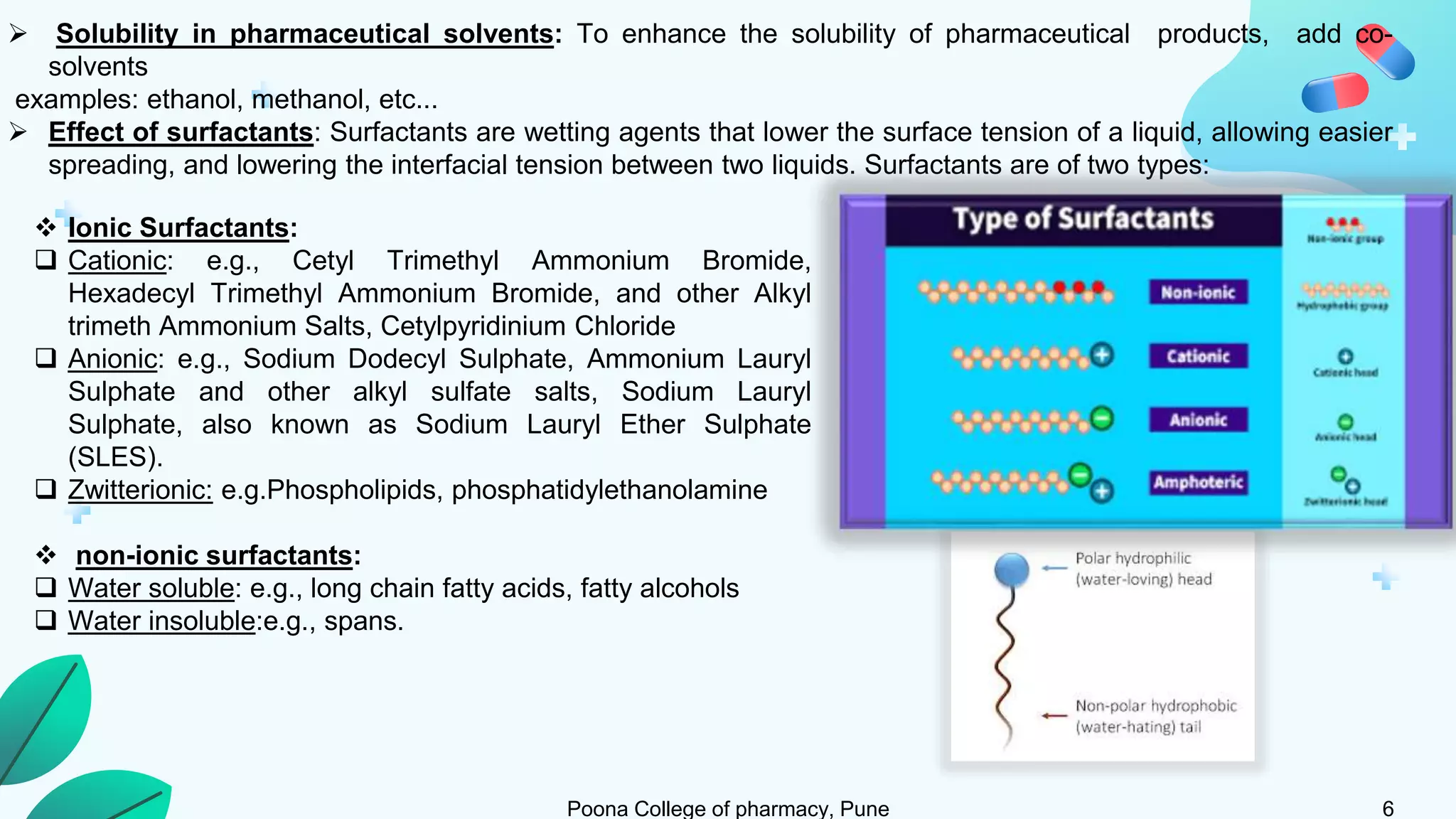
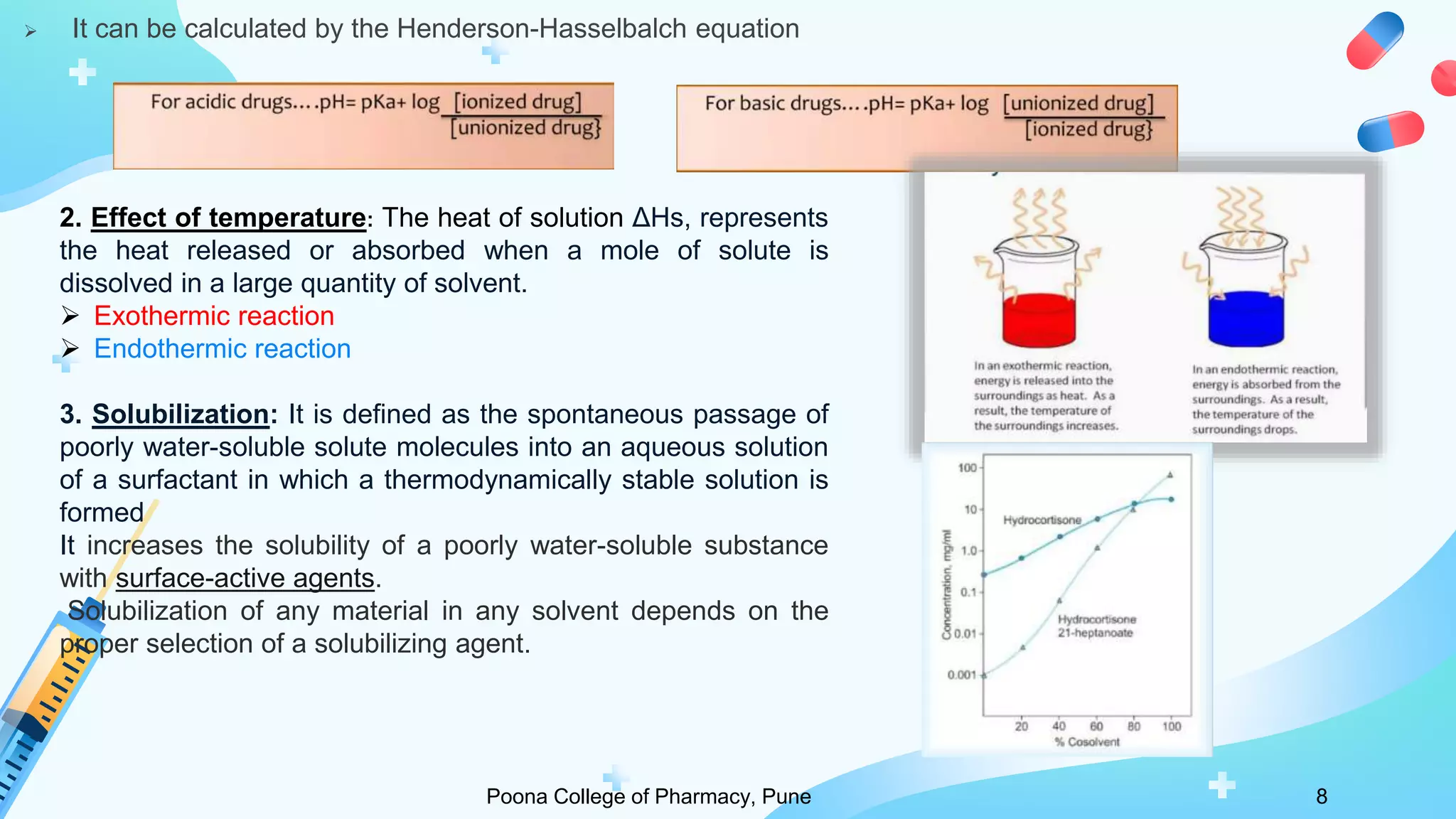
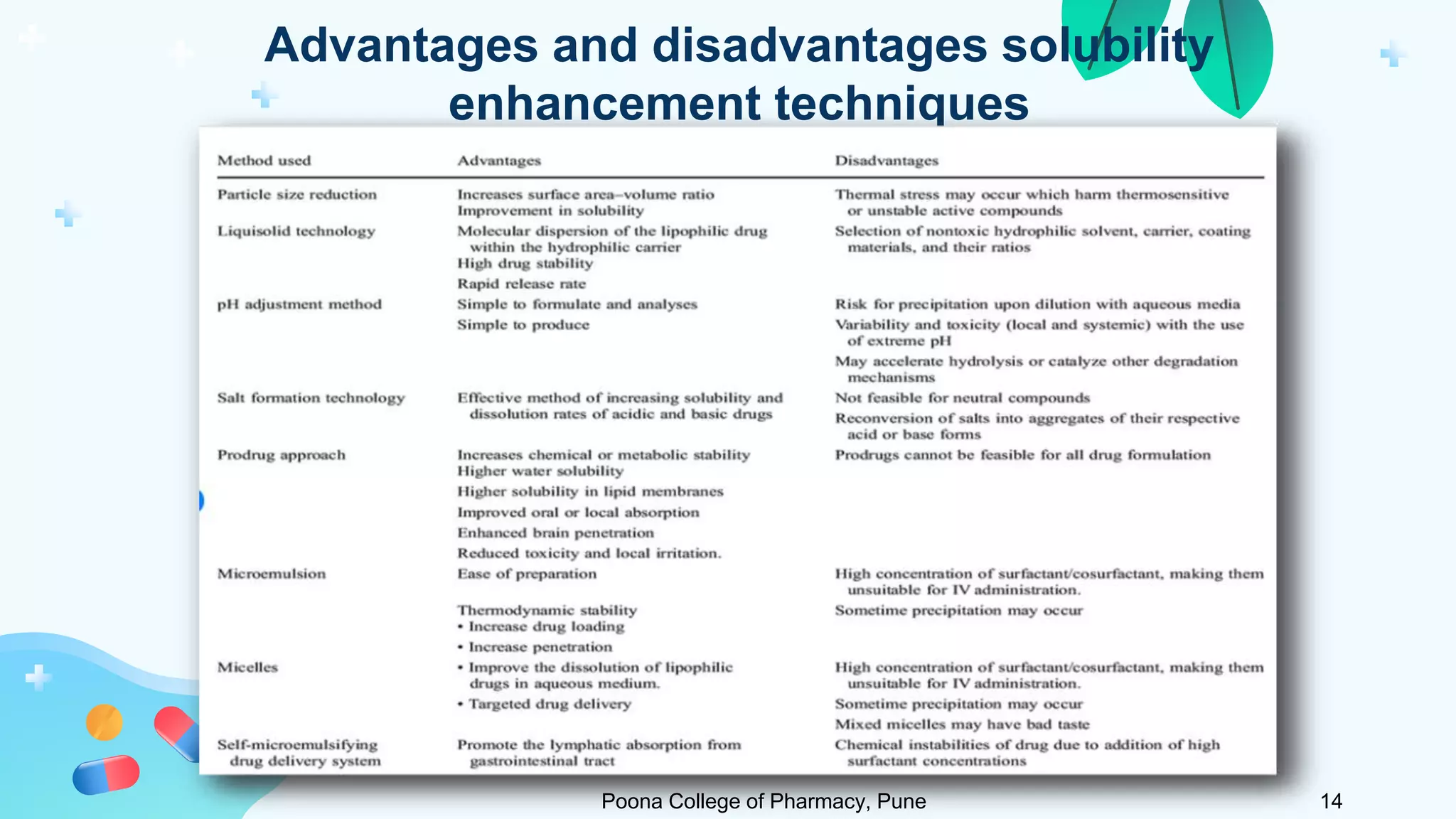
2) Solubility studies include determining intrinsic solubility, pH effects, solubility in solvents and with surfactants. Methods to enhance solubility include changing pH, temperature, adding co-solvents or surfactants.
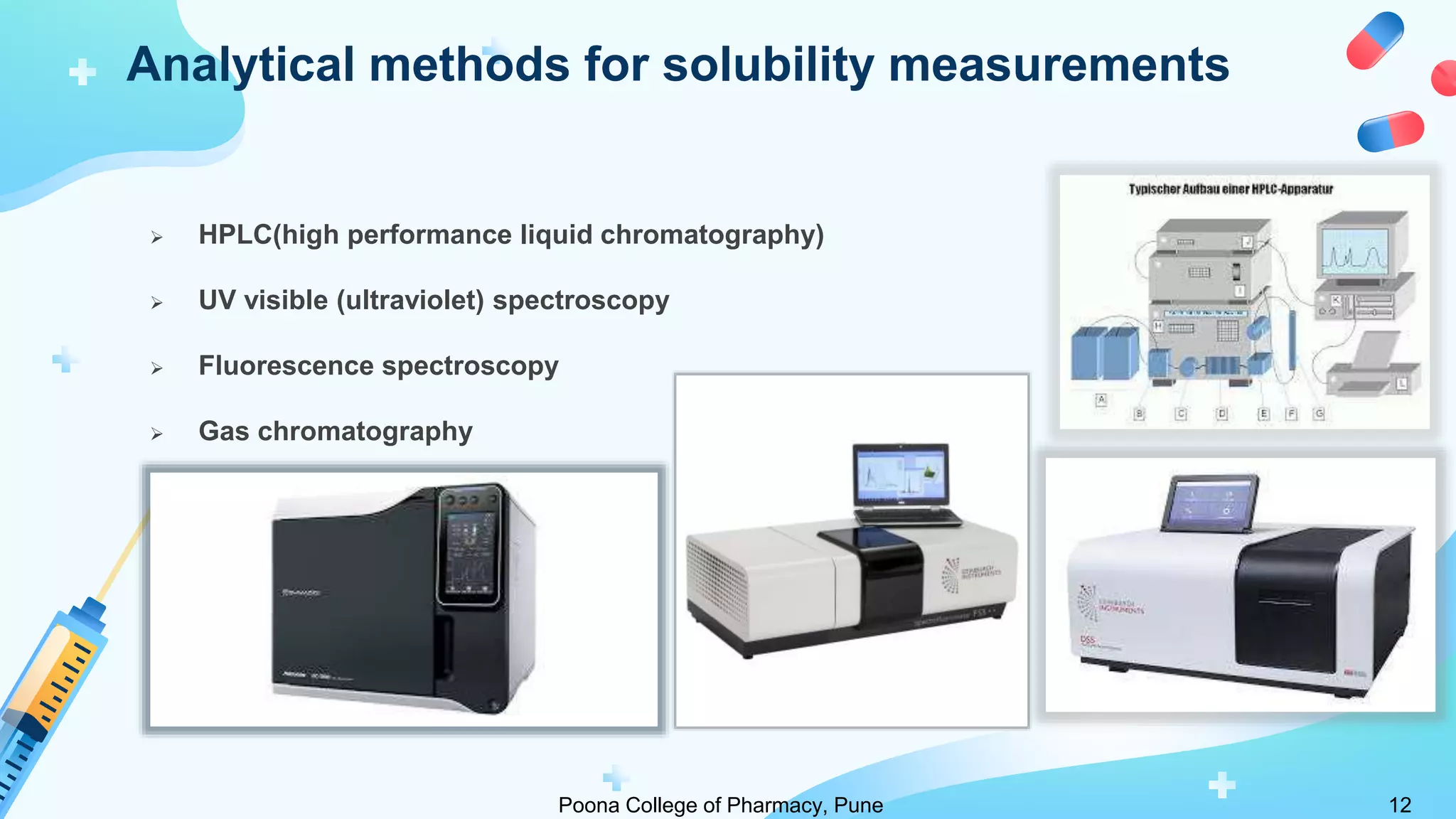
3) The shake flask method is commonly used to determine drug solubility but analytical methods like HPLC provide direct, sensitive analysis of drug concentrations in solutions. Solubility data is important for preformulation studies