The document presents various techniques for enhancing solubility in pharmaceutical chemistry, outlining the importance of solubility for drug bioavailability and therapeutic effectiveness. It covers factors affecting solubility and describes methods including physical and chemical modifications, as well as the use of surfactants and supercritical fluid processes. Additionally, it lists various solubility enhancers and examples of drugs that have been targeted for solubility improvement.


![Introduction
‘Solubility’ is defined as maximum amount of solute that can be dissolved in a
given amount of solvent to form a homogenous system at specified
conditions.[1]
In quantitative terms it is concentration of dissolved solute in a saturated
solution at a specific temperature.[5]
In qualitative terms it means continuous interaction of two or more compound
to form one phase.
The solubility of a drug is represented through various concentration
expressions such as parts, percentage, molarity, molality, mole fraction.[1]
3](https://image.slidesharecdn.com/solubilityenhancementtechniques-230608022943-e32b571b/85/solubility-EnhancementTechniques-pptx-3-320.jpg)
![USP and BP Solubility Criteria 4
Descriptive terms Parts of solvent required for the
one part of solute[3]
Very soluble Less than 1
Freely soluble From 1 to 10
Soluble From10 to 30
Sparingly soluble From 30 to 100
Slightly soluble From 100 to 1000
Very slightly soluble From 1000 to 10,000
Insoluble More than 10,000](https://image.slidesharecdn.com/solubilityenhancementtechniques-230608022943-e32b571b/85/solubility-EnhancementTechniques-pptx-4-320.jpg)
![Need of Solubility
Therapeutic effectiveness of a drug depends up on the bioavailability
and ultimately upon the solubility of drug molecule.[4]
It is important parameter to achieve desired concentration of drug in
systemic circulation for pharmacological response to be shown.
Any drug to be absorbed must be soluble or present in the form of an
aqueous solution at the site of absorption.[4]
5](https://image.slidesharecdn.com/solubilityenhancementtechniques-230608022943-e32b571b/85/solubility-EnhancementTechniques-pptx-5-320.jpg)

![Enhancement Techniques
When the solubility of substances in aqueous media is limited, here are
various techniques to improve the solubility of poorly soluble drug.[4]
7
Solubility
Enhancement
techniques
• Physical modification
• Chemical modification
• pH adjustment
• Supercritical fluid process](https://image.slidesharecdn.com/solubilityenhancementtechniques-230608022943-e32b571b/85/solubility-EnhancementTechniques-pptx-7-320.jpg)
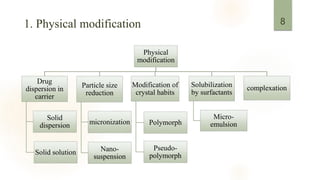
![Particle size reduction:
Micronization:
Increase dissolution – increase S.A. e.g.: progesterone, fenofibrate.
Nanosuspension:
Used to drugs that are insoluble in water & oils. Nanosuspension is biphasic system
which consists nano size particle in aqueous vehicle.[2]
Drug dispersion in carrier:
Solid solution:
Blend of two crystals to form one new phase.
Solid dispersion:
Hydrophobic drug + hydrophilic matrix. Thermal decomposition can be prevented.
9](https://image.slidesharecdn.com/solubilityenhancementtechniques-230608022943-e32b571b/85/solubility-EnhancementTechniques-pptx-9-320.jpg)
![Modification of crystal habits:
Based on polymorphism as different polymorphs of drugs are chemically
identical, but they exhibit different physicochemical properties.[2]
Order for dissolution of different solid forms of drug is:
Amorphous >Metastable polymorph >Stable polymorph
Solubility by surfactants:
The addition of surfactants decreases the surface tension and increase the
solubility of the lipophilic drugs.[1]
Complexation:
Drugs have been complexed with cyclodextrins to improve water solubility and
drug stability.[3]
10](https://image.slidesharecdn.com/solubilityenhancementtechniques-230608022943-e32b571b/85/solubility-EnhancementTechniques-pptx-10-320.jpg)
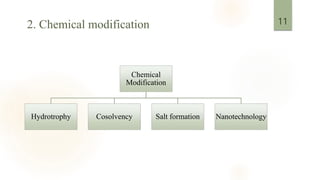
![3. pH adjustment
To access the solubility by this approach, the buffer capacity and tolerability of
the selected pH are important to consider.[4]
Excipients act as alkalizing agents may increase the solubility of weekly basic
drugs.[4]
Supercritical fluids (SCFs) can dissolve nonvolatile solvents, with the critical
point of carbon dioxide.[5]
It is safe, environmentally friendly, and economical.
Low operating conditions are required.
12
4. Supercritical fluid process](https://image.slidesharecdn.com/solubilityenhancementtechniques-230608022943-e32b571b/85/solubility-EnhancementTechniques-pptx-12-320.jpg)



