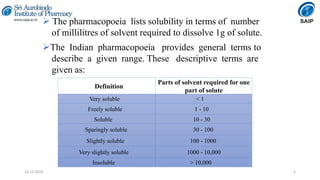
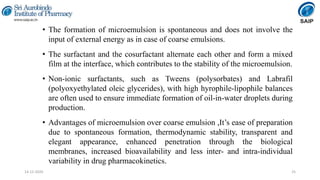
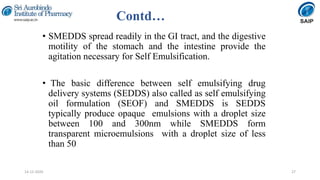
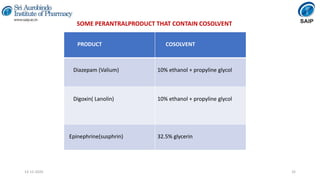
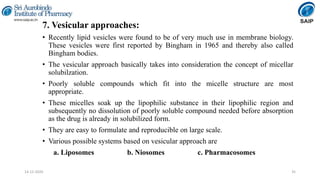
This document discusses various techniques for enhancing the solubility of poorly soluble drugs, which is an important consideration in drug development and formulation. It begins by defining solubility and explaining the importance of solubility for drug bioavailability. Some key techniques discussed include particle size reduction through micronization or supercritical fluid processing, modifying the drug's crystal structure through polymorphism or amorphization, forming solid dispersions or complexes with carriers like cyclodextrins, and chemical modifications like salt formation or prodrugs. The document provides an overview of the various physical, chemical, and other approaches to improving a drug's solubility.