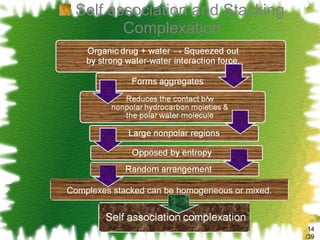
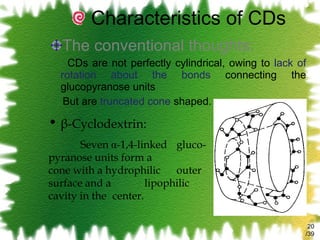
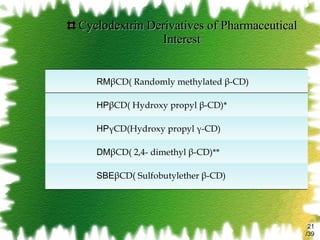
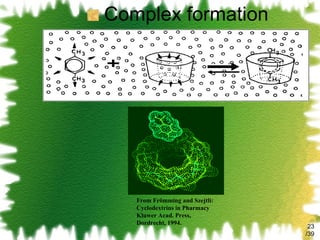
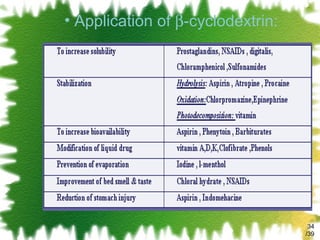
The document discusses methods for enhancing the solubility of poorly soluble drugs, focusing on pH alteration and complexation techniques. It explains how solubility can be influenced by pH for both weak acids and bases and details the use of cyclodextrins in improving drug delivery and stability. Additionally, it outlines various characterization methods for inclusion complexes and highlights the advantages of cyclodextrins over organic solvents in pharmaceutical applications.




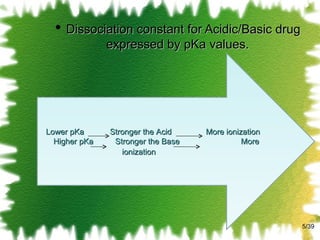


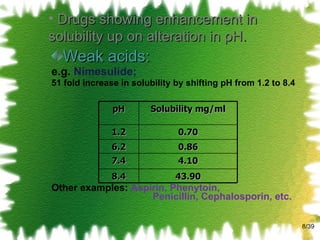

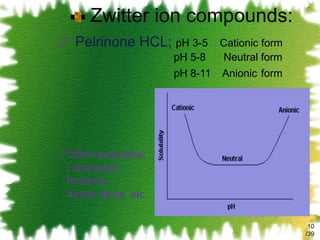


![The mathematical description for the equilibrium constant of a 1:1 complex K 1:1 = [SL] / [S] [L] K 1:1 is defined as equilibrium constant/ Stability constant/ Complexation constant. /39](https://image.slidesharecdn.com/2-solubillityenhancementbyphandcomplexation-111002011824-phpapp02/85/solubility-enhancement-by-pH-change-complexation-13-320.jpg)