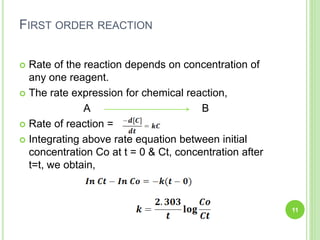
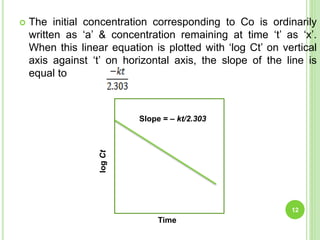
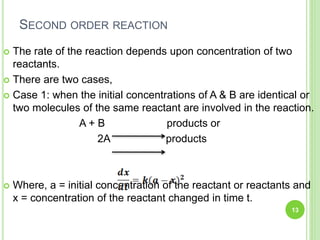
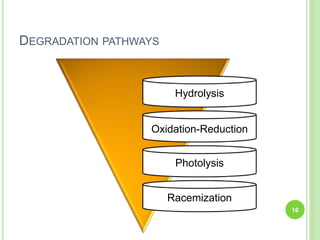
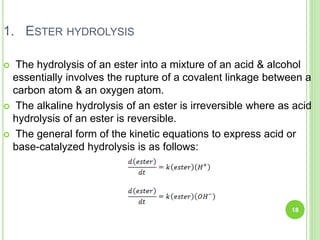
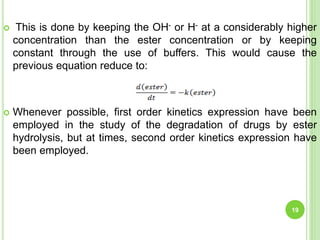
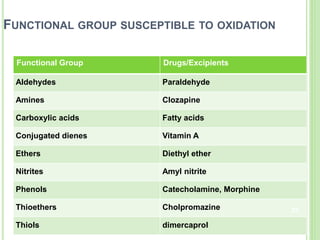
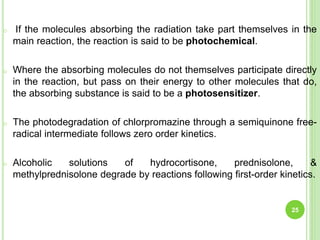
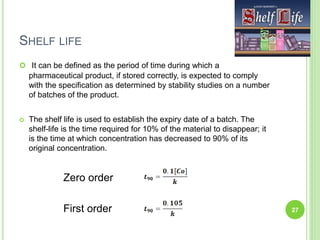
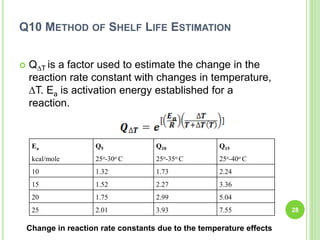
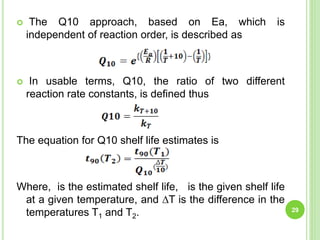
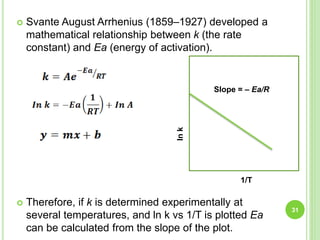
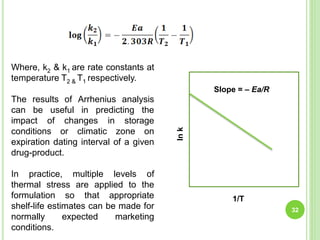
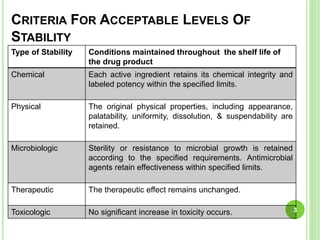
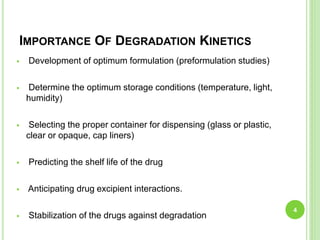
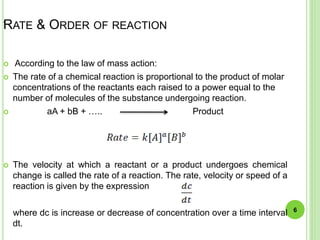
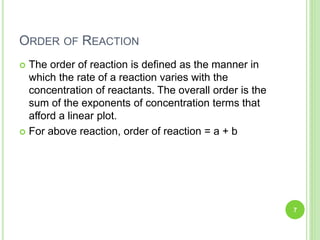
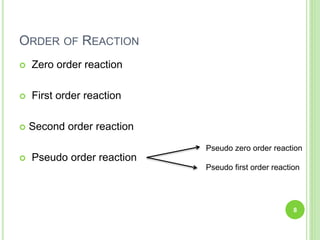
The document discusses drug stability and degradation kinetics. It defines drug stability as the ability of a pharmaceutical dosage form to maintain its physical, chemical, therapeutic and microbial properties during storage and usage. The main criteria for acceptable stability are that each active ingredient retains its chemical integrity and potency. Degradation kinetics aims to predict a drug's intrinsic stability by determining the order of degradation reactions and their rate constants. Common degradation pathways include hydrolysis, oxidation, photolysis and racemization. The Q10 method can be used to estimate shelf life based on a drug's activation energy.



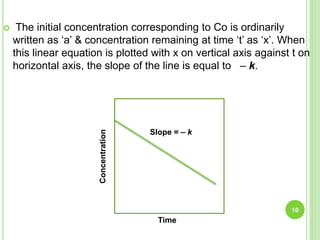
![ZERO ORDER REACTION
The rate of the reaction doesn't depend on concentration of the reagent(s).
The rate expression for chemical reaction,
A B
Rate of reaction = - d[C]/dt = k
where, [C] indicates decreasing concentration of reagent & k indicates rate
constant.
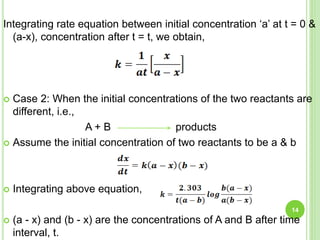
Integrating of rate equation between initial concentration Ao at t = 0 & At,
concentration after t = t we obtain,
9](https://image.slidesharecdn.com/degradationkinetics2-141231080242-conversion-gate01/85/Degradation-kinetics-9-320.jpg)