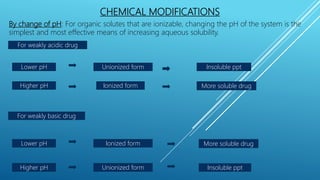
The document discusses solubility enhancement techniques crucial for improving the water solubility of poorly soluble drugs. It categorizes these techniques into physical modifications, chemical modifications, and other methods, detailing various strategies such as particle size reduction, pH adjustments, and the use of supercritical fluids. The document emphasizes the importance of selecting appropriate solubility enhancers based on the drug molecule and administration route.