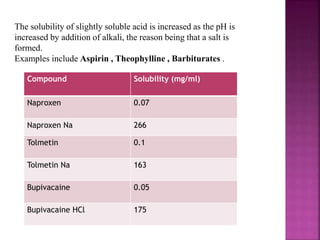
The document discusses various techniques to improve the solubility of poorly soluble drugs, including salt formation, co-solvency, and particle size reduction. It focuses on using salt formation between flurbiprofen and tris(hydroxymethyl)aminomethane to increase solubility. Analytical techniques like DSC, TGA, HPLC, and UV were used to characterize the flurbiprofen-tris salt and showed improved solubility over flurbiprofen alone. The conclusion states that increasing water solubility of insoluble drugs is important for developing effective dosage forms and delivering drugs to the absorption site.

















![The role of counter-ion in salt formation to improve the
solubility of poorly water soluble drugs in the case of
Flurbiprofen and Tris(hydroxymethyl)amino methane
• This study shows the role of salt formation in
improving the solubility and dissolution rates of
Flurbiprofen, which is one of the poorly water
soluble drugs.
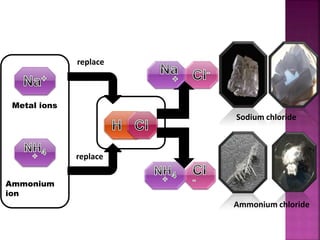
• Tris[hydroxymethyl]aminomethane was used as
a counter-ion and the Flurbiprofen-Tris salt was
crystallized from acetonitrile as solvent.](https://image.slidesharecdn.com/neethuppt-140929003520-phpapp02/85/Neethu-ppt-23-320.jpg)
![ Flurbiprofen: is a non-steroidal anti-inflammatory drug
(NSAID) that readily forms carboxylic acid salts. It is
administered for its anti-inflammatory, antipyretic, analgesic
effects, and to inhibit intraoperative mitosis.
Tris[hydroxymethyl]aminomethane: was used as a
counter-ion .
Acetonitrile: is a polar solvent that used to crystallize the
Flurbiprofen-Tris salt .
MSc presentation project 24
EXAMPLE :](https://image.slidesharecdn.com/neethuppt-140929003520-phpapp02/85/Neethu-ppt-24-320.jpg)
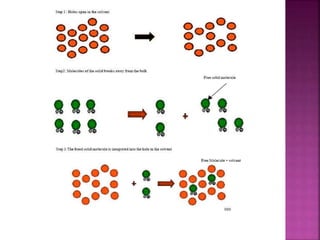
![The Flurbiprofen and Tris [hydroxy methyl]amino methane were
combined to prepare an soluble salt, which was then precipitated
and yield the final product.
MSc presentation project
- +
3
Acetonitrile
Flurbiprofen
Tris[hydroxymethy
l]aminomethane
The
equation
reaction
reactants product
Flurbiprofen-
Tris salt
Solvent](https://image.slidesharecdn.com/neethuppt-140929003520-phpapp02/85/Neethu-ppt-25-320.jpg)