This document describes Richardt's experiment to measure the ratio of specific heats (γ) of air. It discusses:
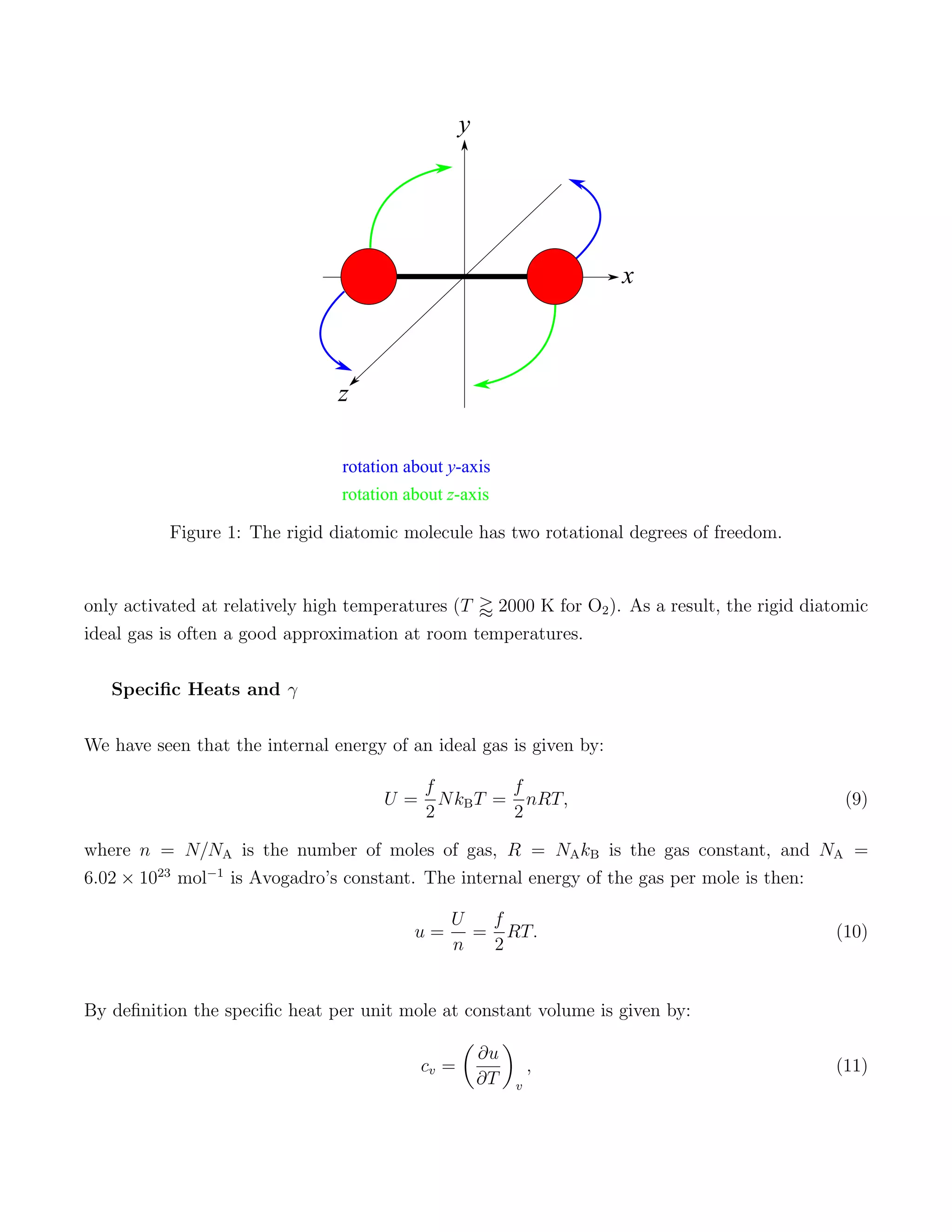
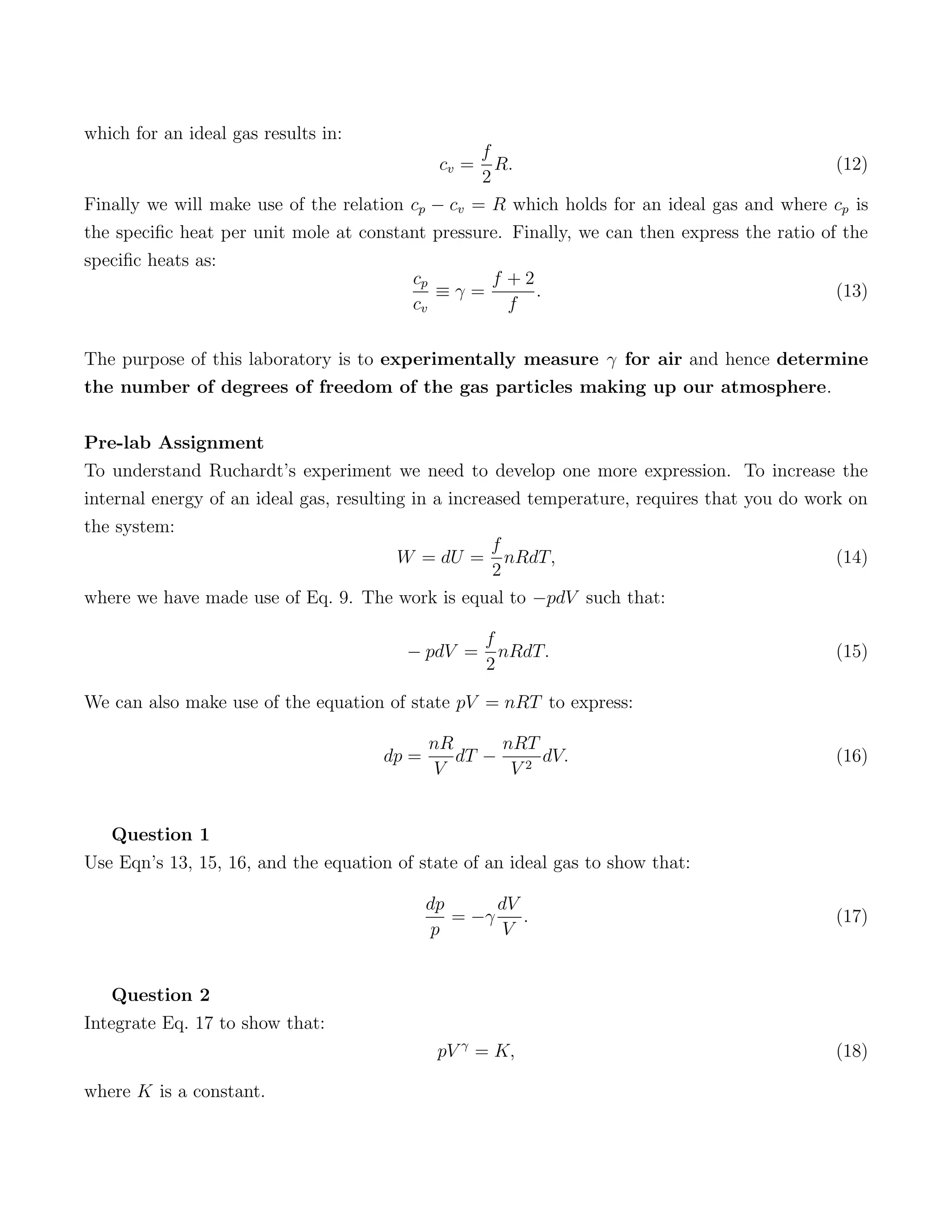
1) The degrees of freedom of ideal gas particles and how this relates to the internal energy and specific heats of different gases. Monatomic gases have 3 translational degrees while diatomic gases can also rotate, giving 5 total degrees of freedom.
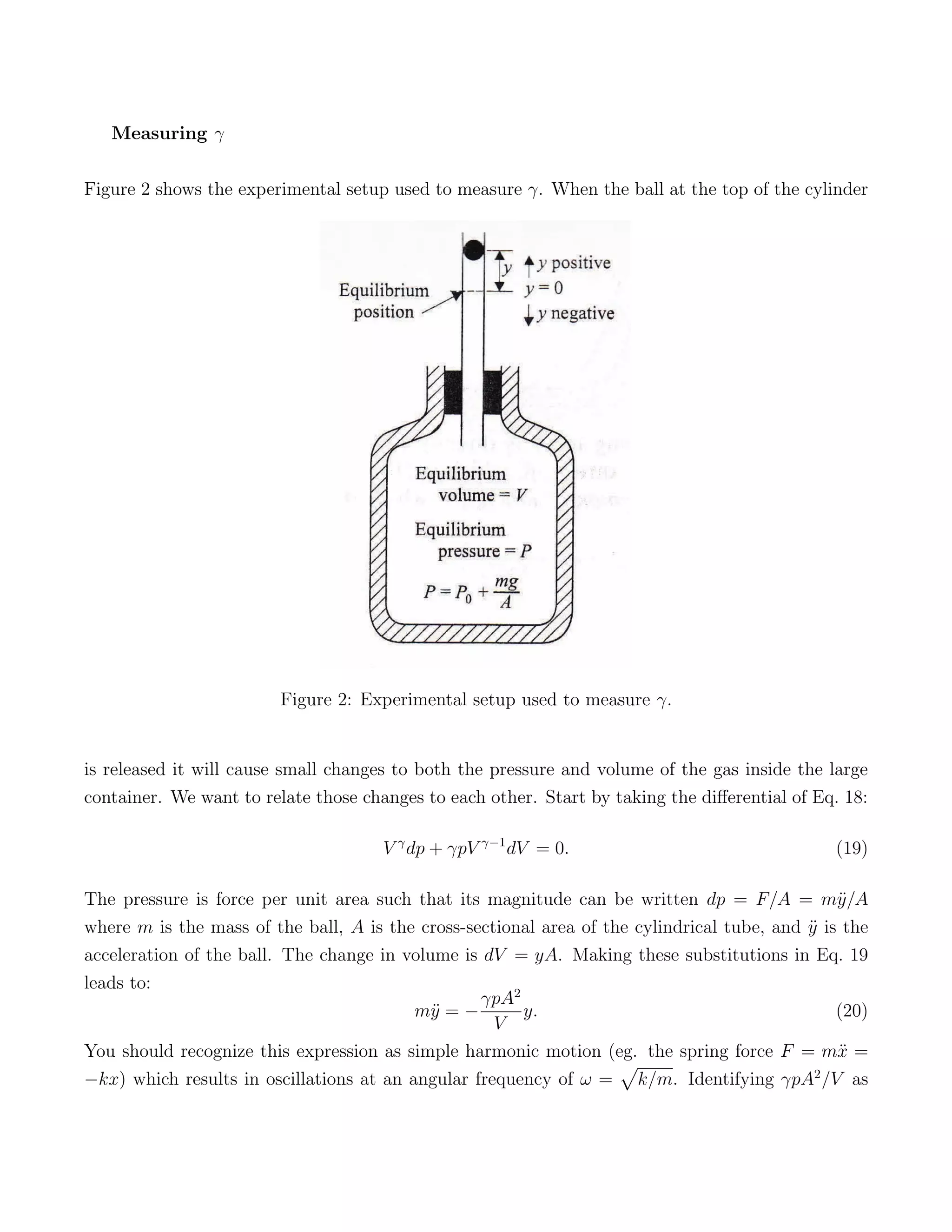
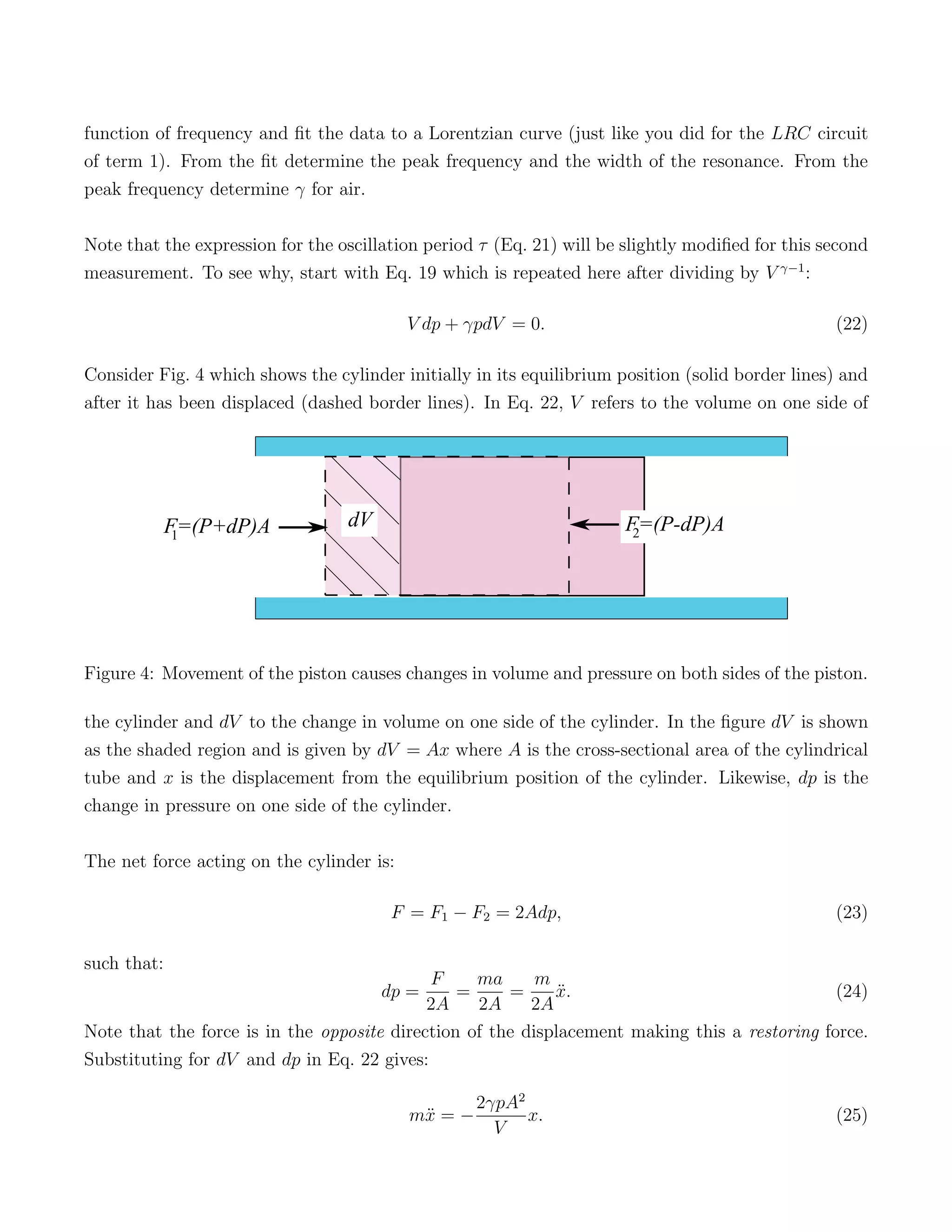
2) The experimental setup uses a ball dropping in a cylinder to cause small pressure and volume changes in the contained gas. The relationship between these changes can be used to determine γ.
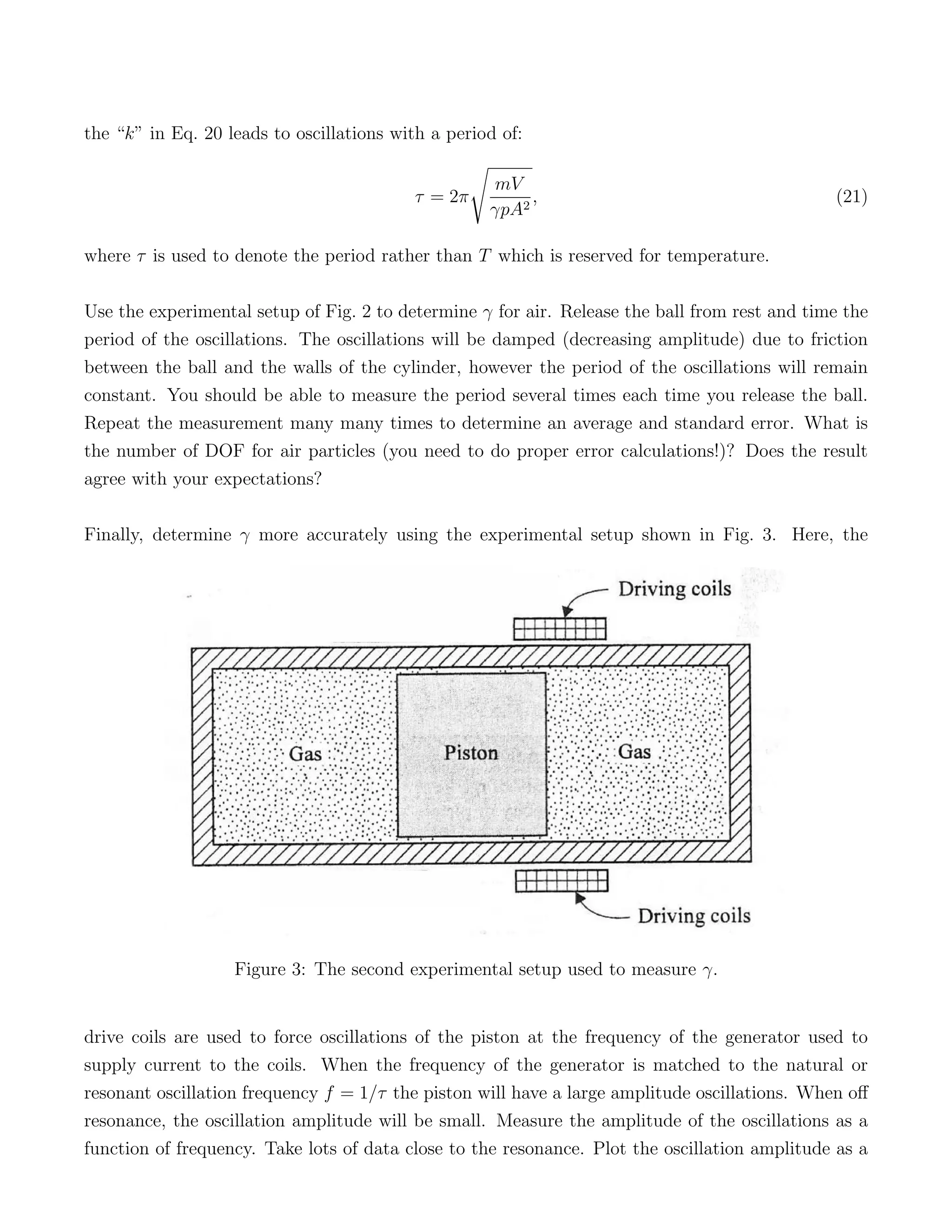
3) Releasing the ball results in simple harmonic motion that allows the measurement of γ, identifying air as a diatomic gas based on it having 5