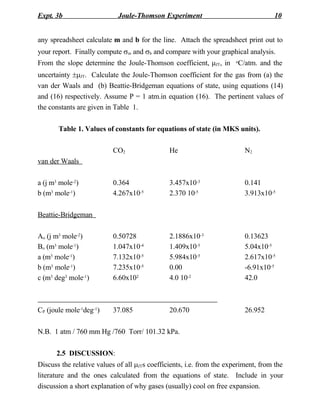
1) The Joule-Thomson experiment measures the temperature change of a gas as it expands freely through a porous plug from high to low pressure.
2) For an ideal gas, the temperature remains constant during expansion (mJT = 0). For real gases, expansion may cause cooling or warming depending on the gas.
3) The experiment determines the Joule-Thomson coefficient (mJT), which is the rate of change of temperature with respect to pressure during constant enthalpy expansion. A positive mJT means cooling during expansion.






![Expt. 3b Joule-Thomson Experiment 8
JT =
2a
RT
−b
(14)
CP
2.2 (b) THE BEATTIE-BRIDGEMAN EQUATION OF STATE:
PV =RT 2 3 (15)
V V V
RC
=RTB 0 − A0 −
T2
=RB 0 bc/T 2
has five adjustable constants Ao, Bo, a,b,c,compared with van der Waals two.
A similar procedure to that used for the van der Waals equation gives the Joule-
Thompson coefficient for the Beattie-Bridgeman equation:-
1 2 Ao 4c 2B b 3 Ao a 5 Bo c
m JT = { - Bo + + 3 +[ o - 2
+ ]P } (16)
CP RT T RT (RT ) RT 4
2.3 EXPERIMENTAL PROCEDURE:
The Joule-Thomson apparatus is shown in Fig. 4. This apparatus will be set up for
you and initial adjustments will not be necessary. Because it takes a long time for the
porous frit to come to a steady thermal state, the gas will be turned on some two
hours prior to the start of the laboratory to ensure that the temperature difference
across the porous frit has attained a constant value. This is indicated by the
constancy of emf of the thermocouple.
Figure 4. Joule-Thomson Apparatus](https://image.slidesharecdn.com/thejoule-thomsonexperiment-111208101256-phpapp01/85/The-joule-thomson-experiment-8-320.jpg)