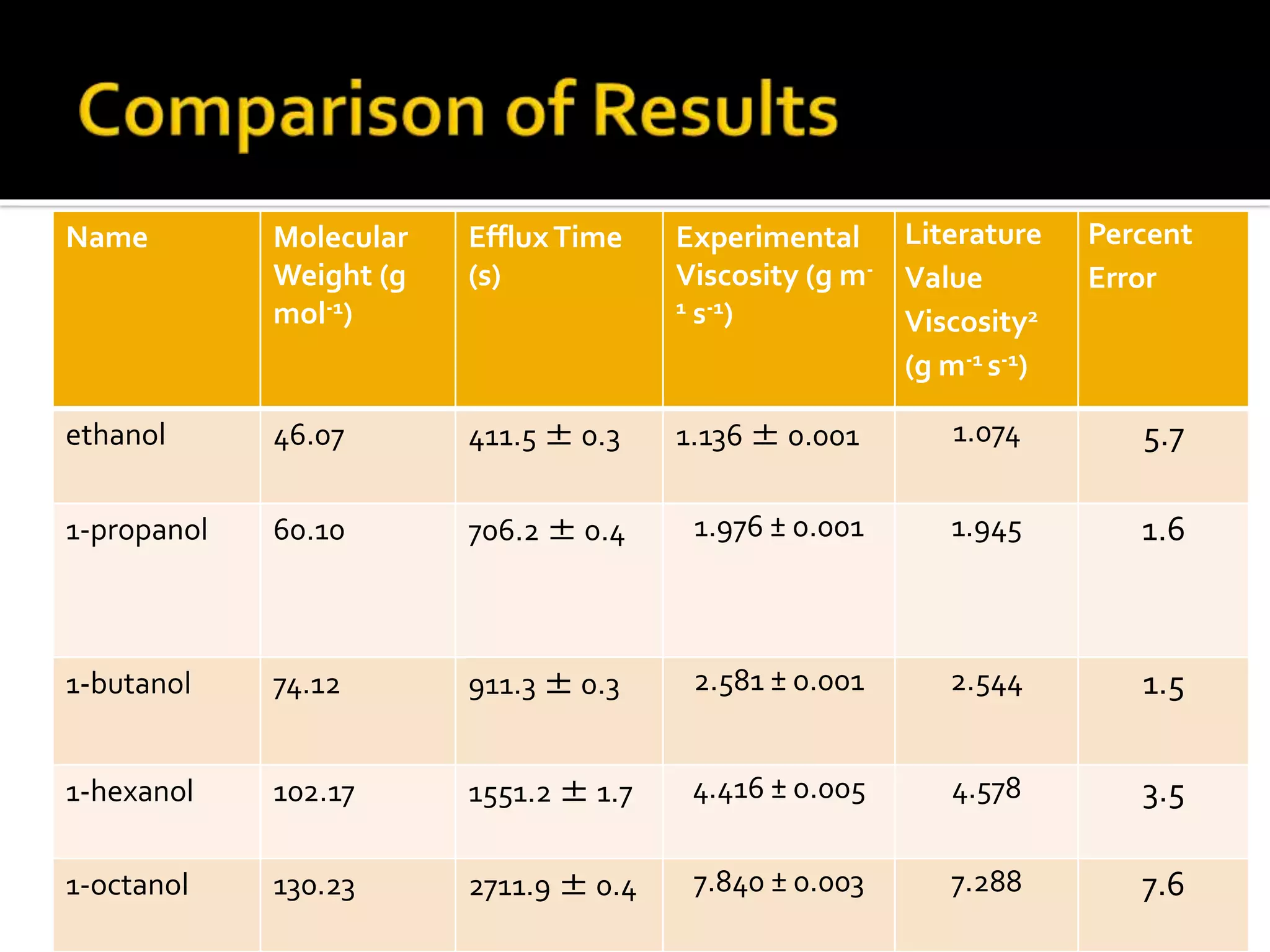
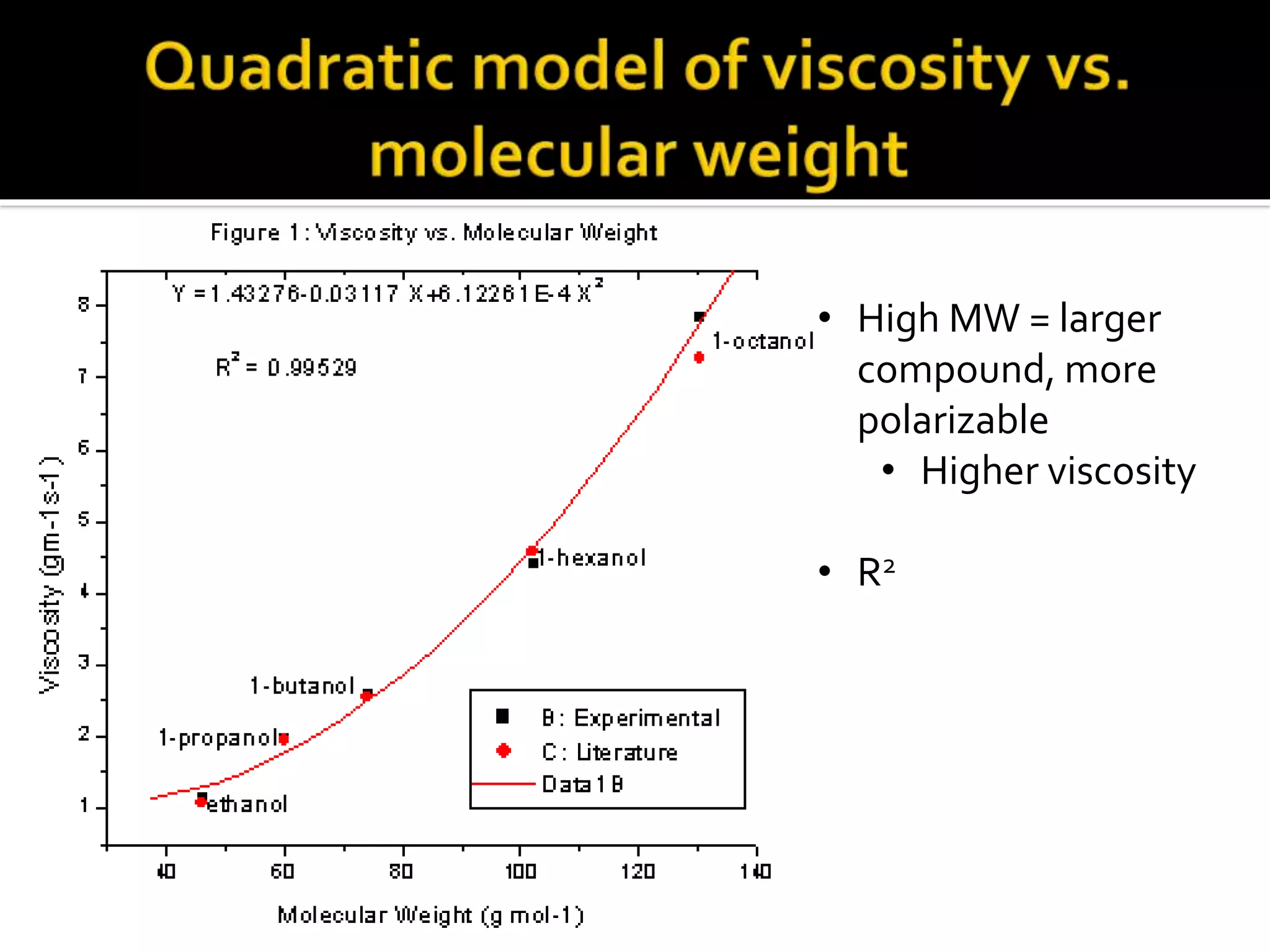
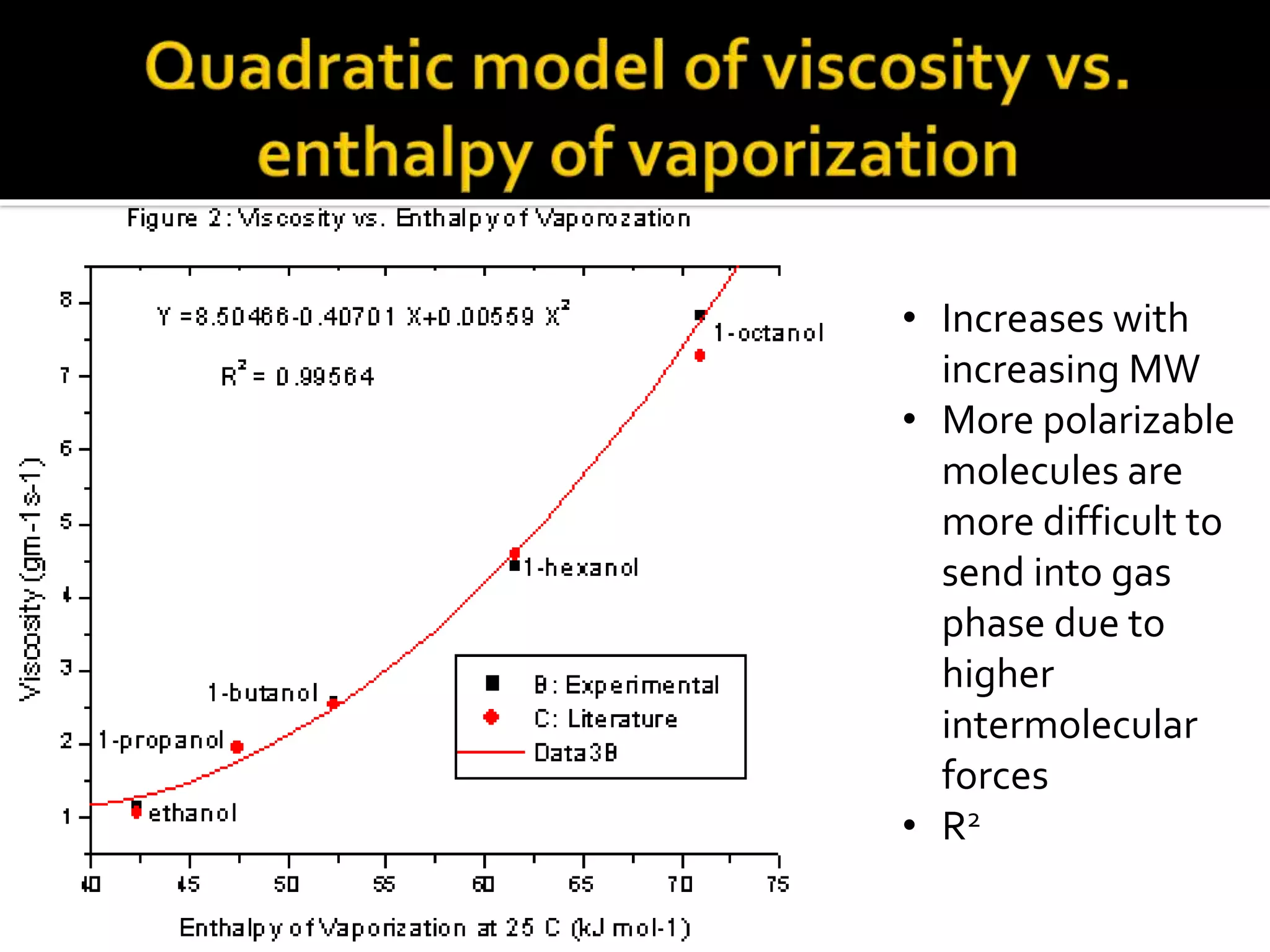
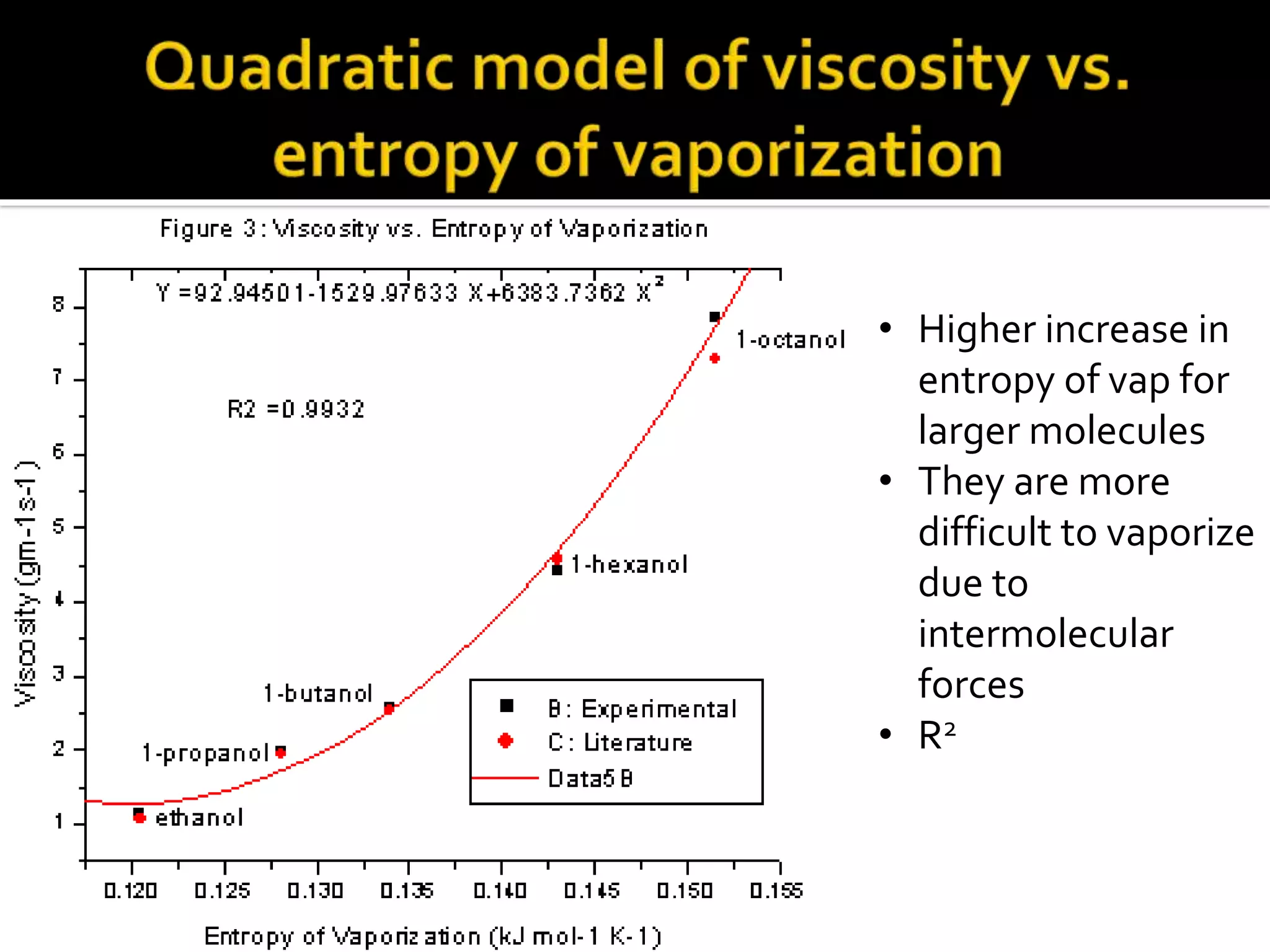
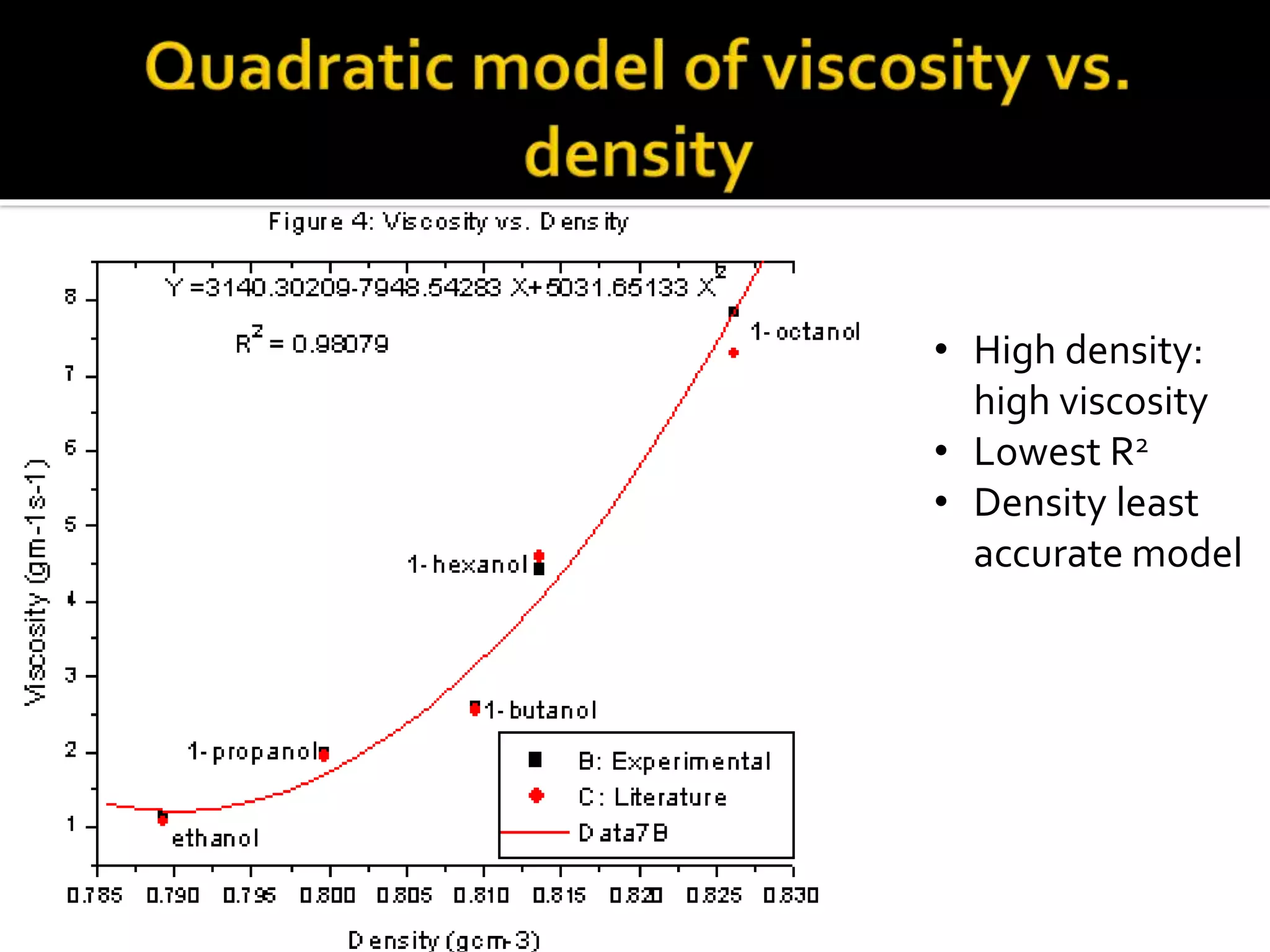
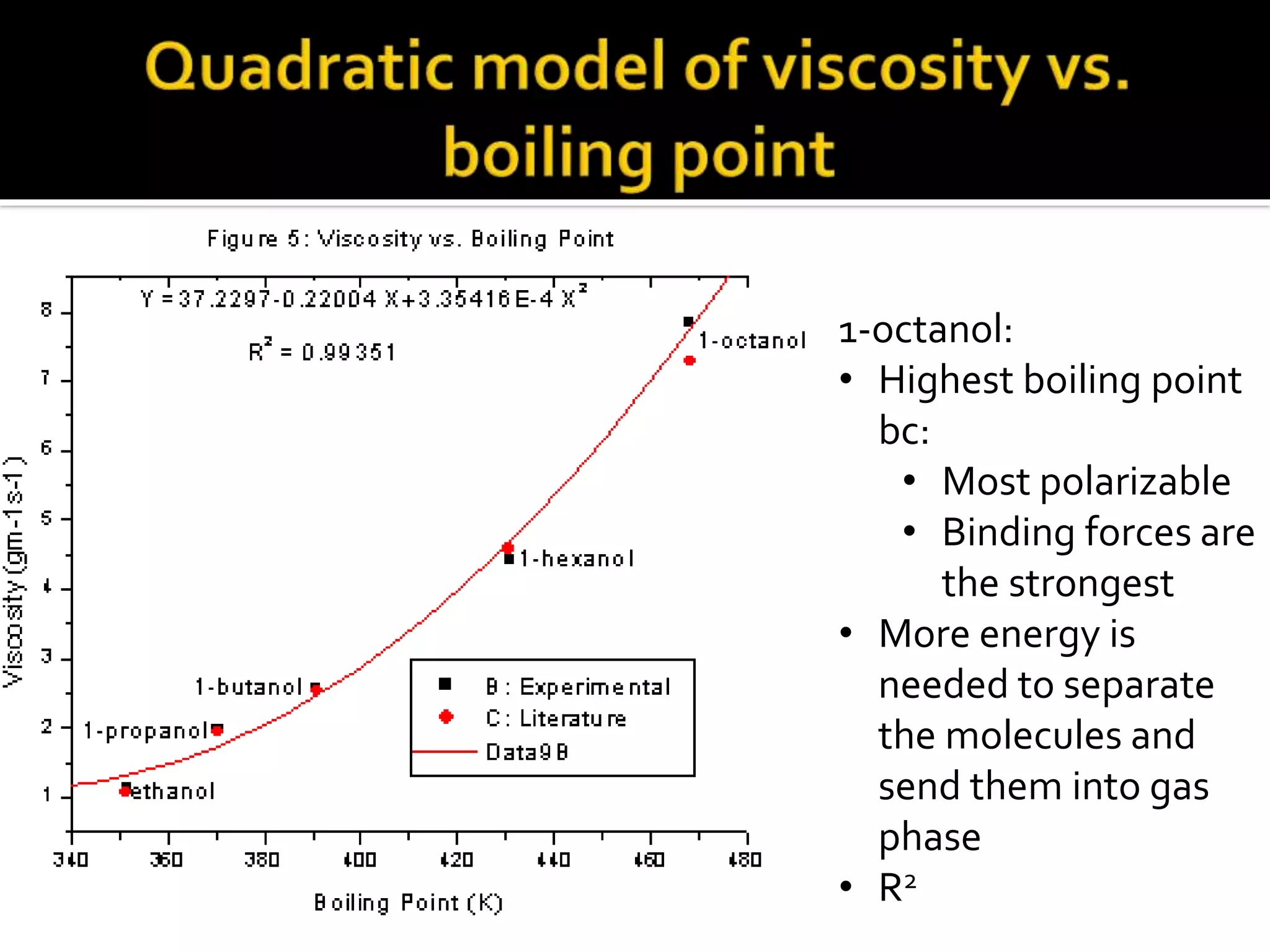
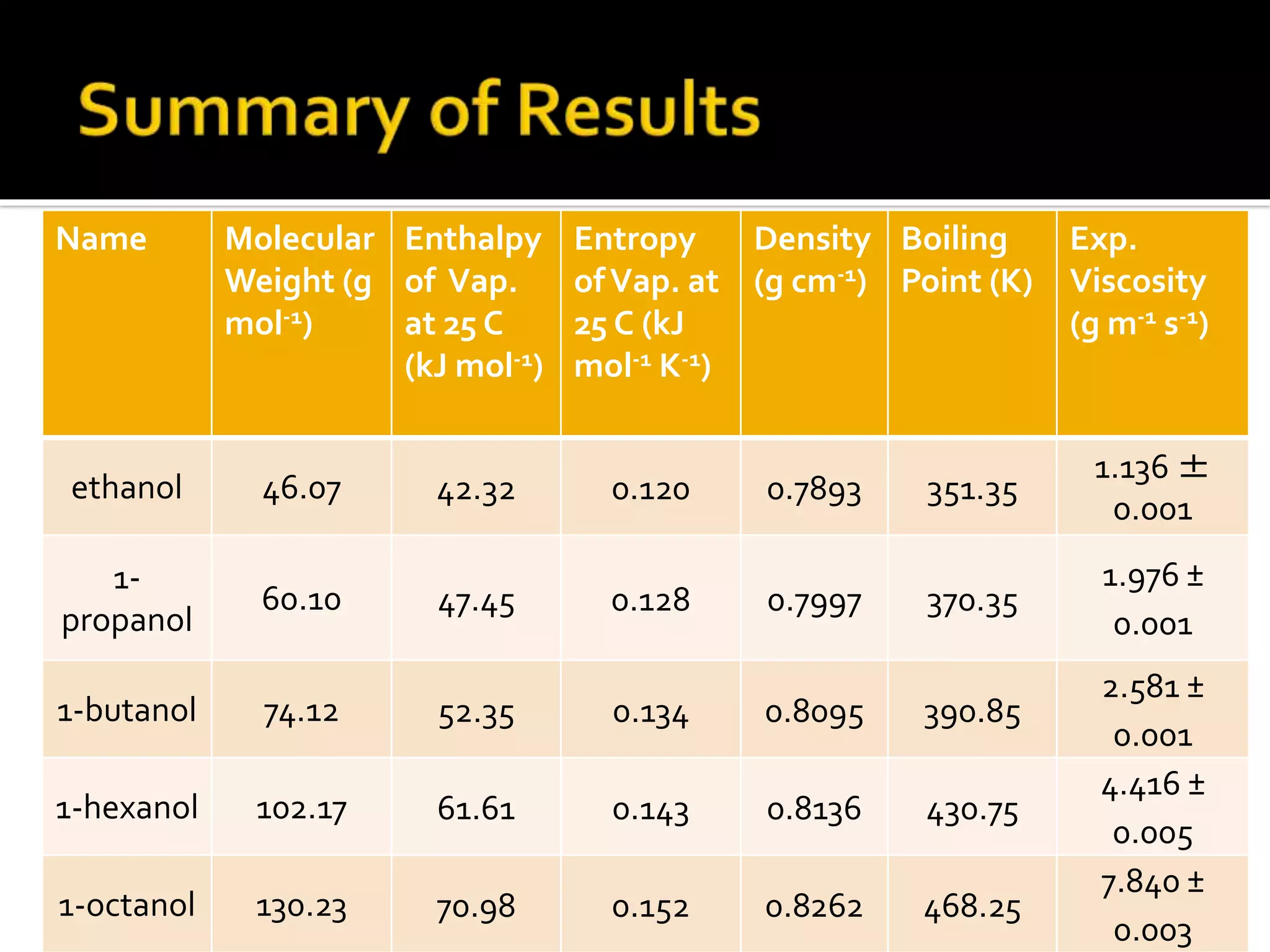
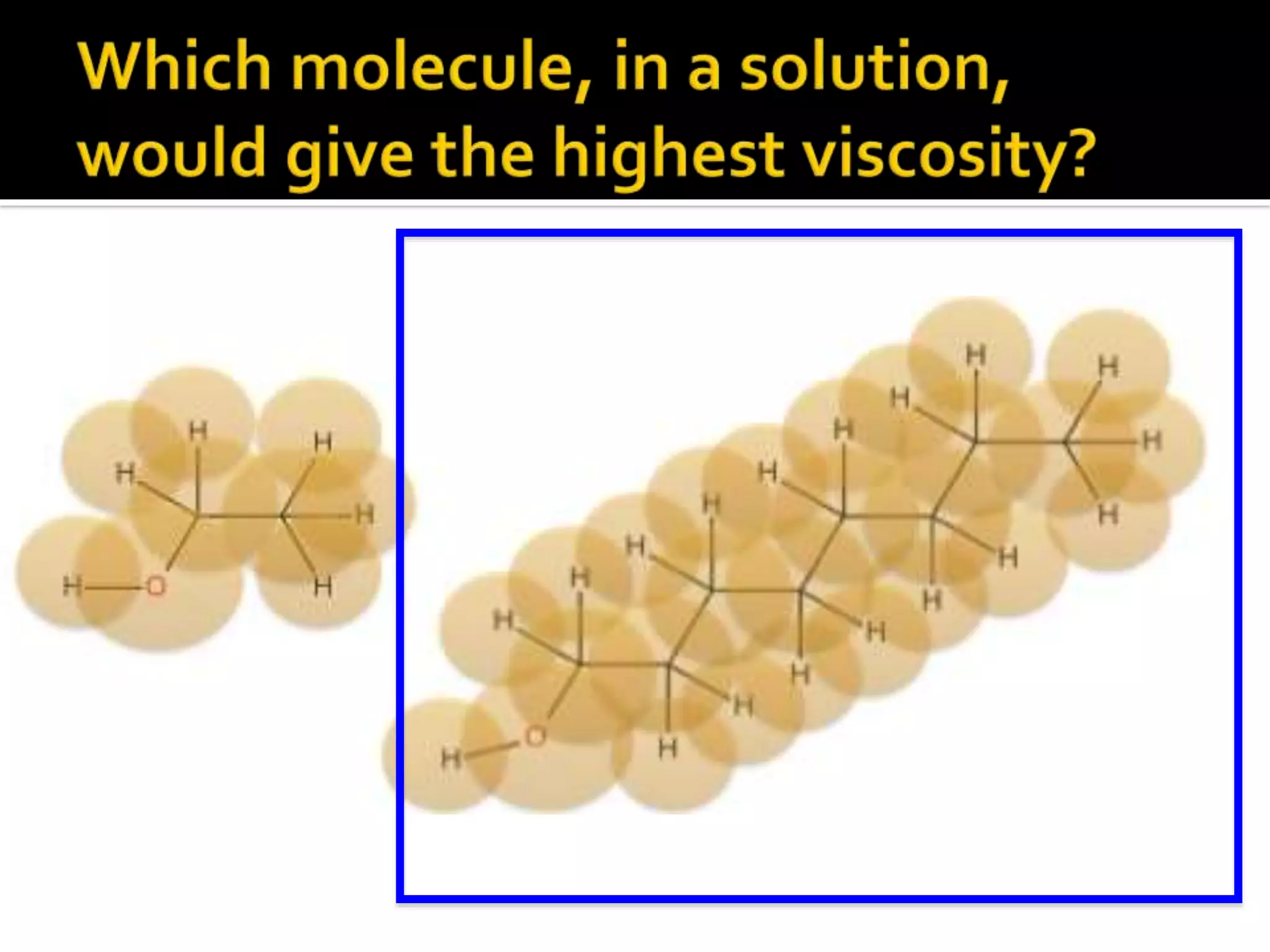
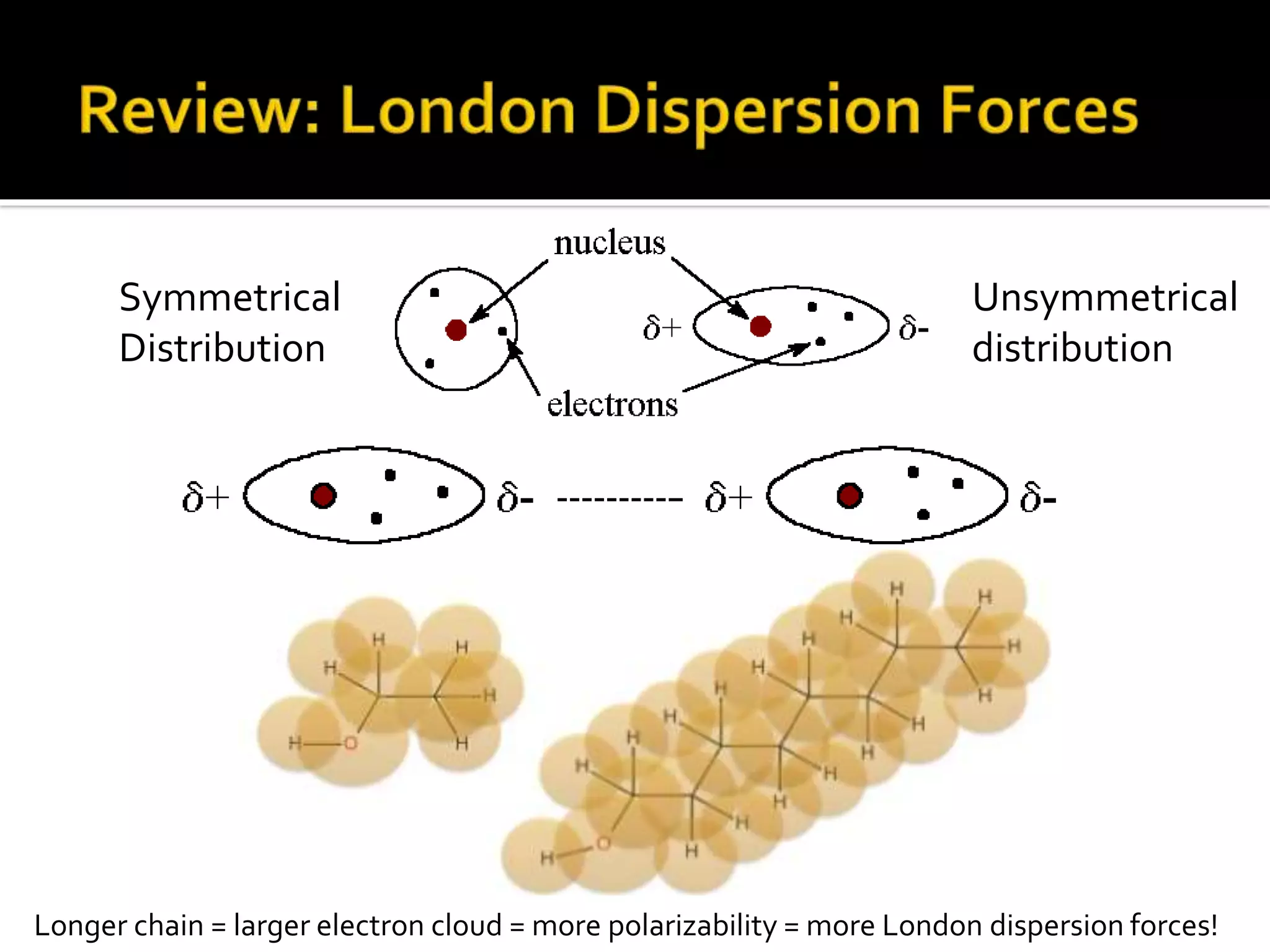
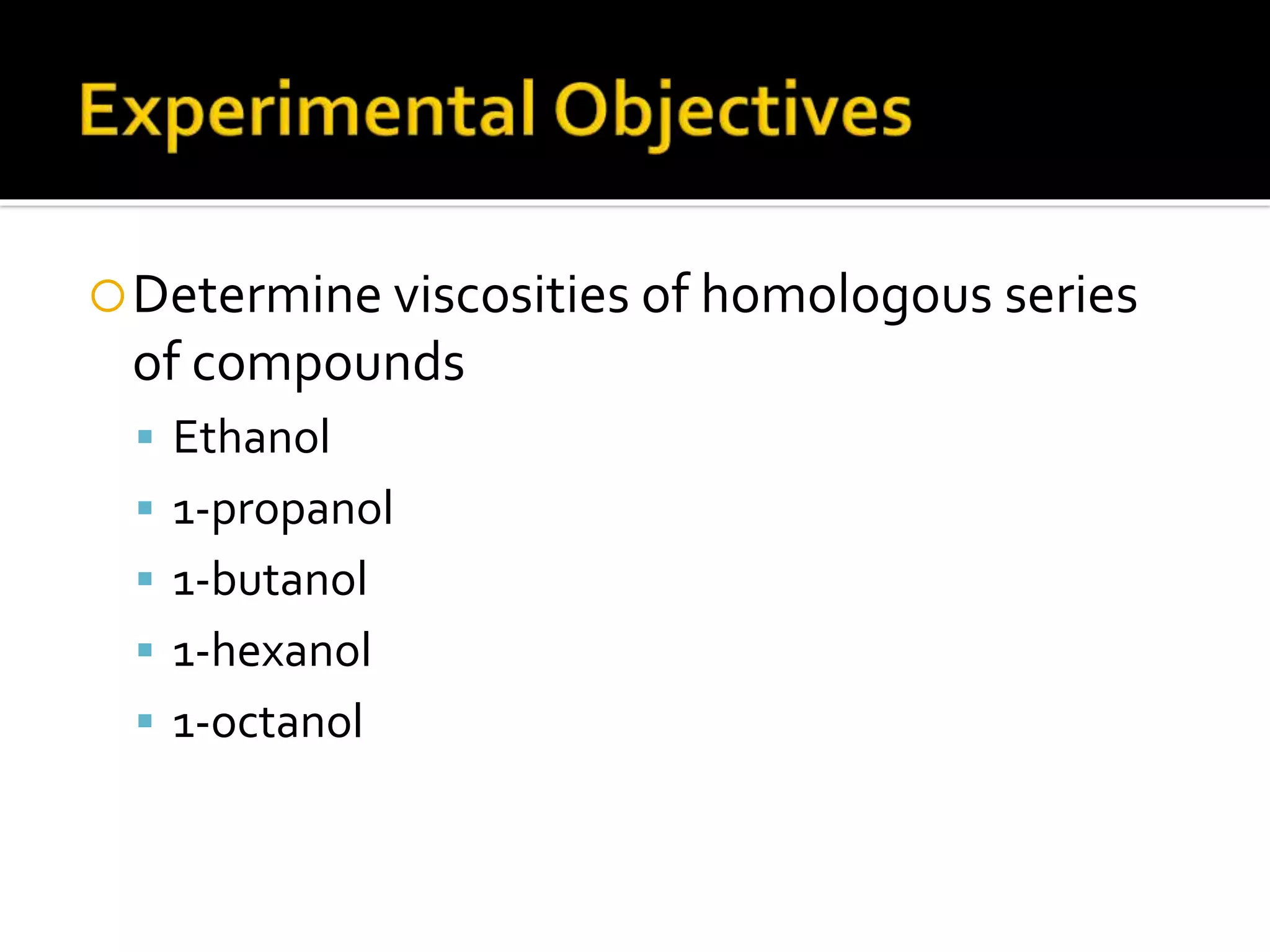
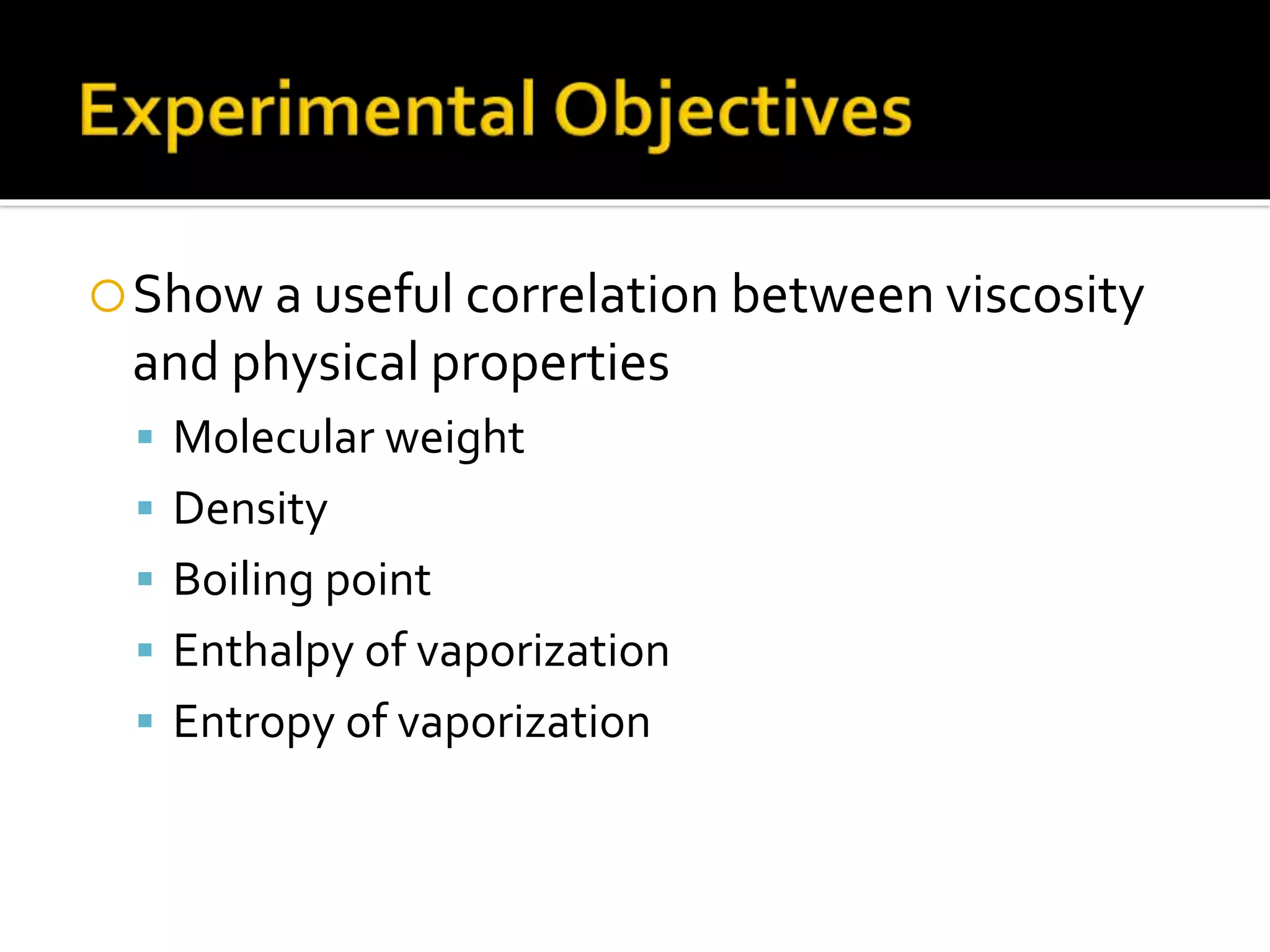
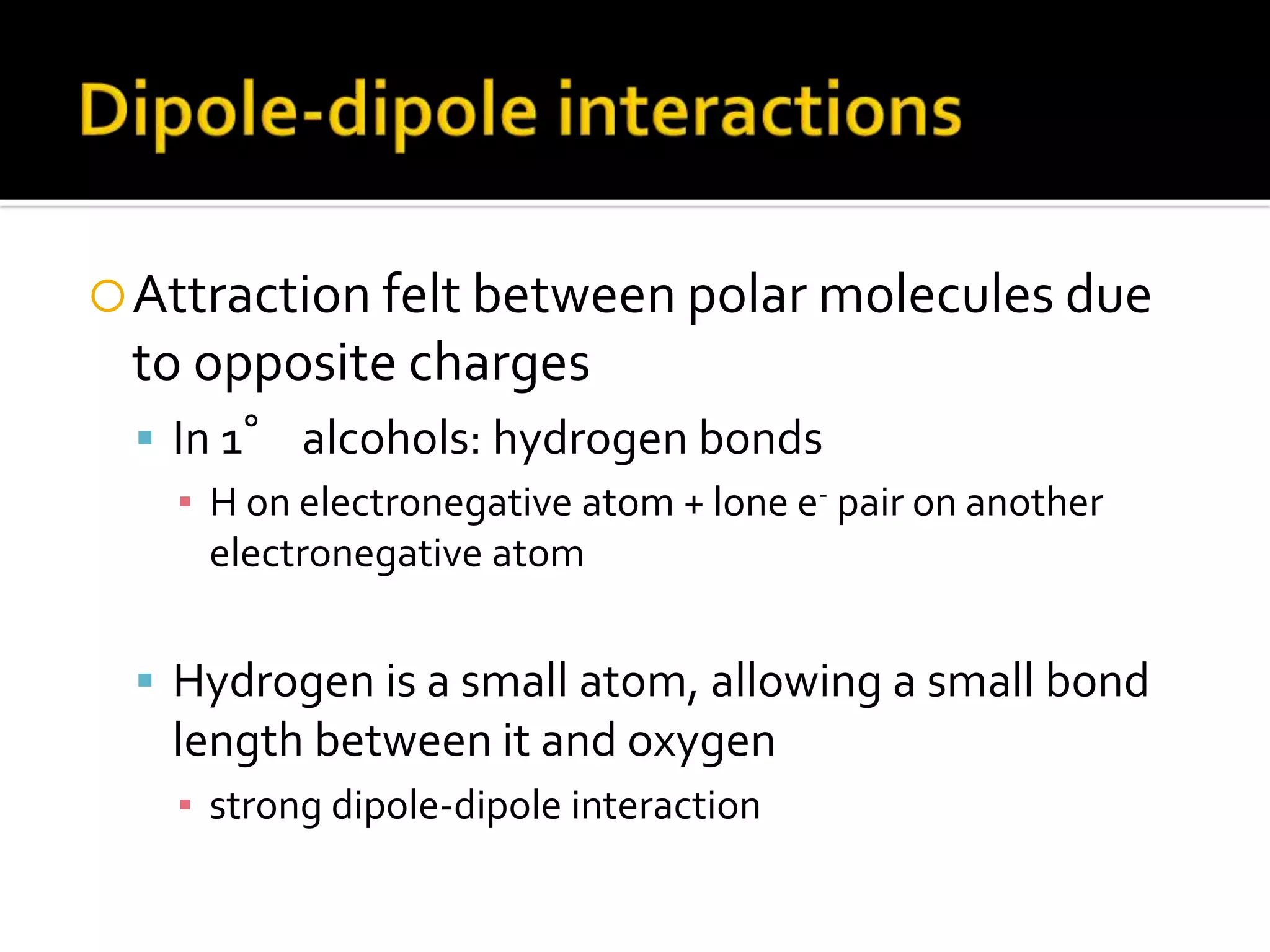
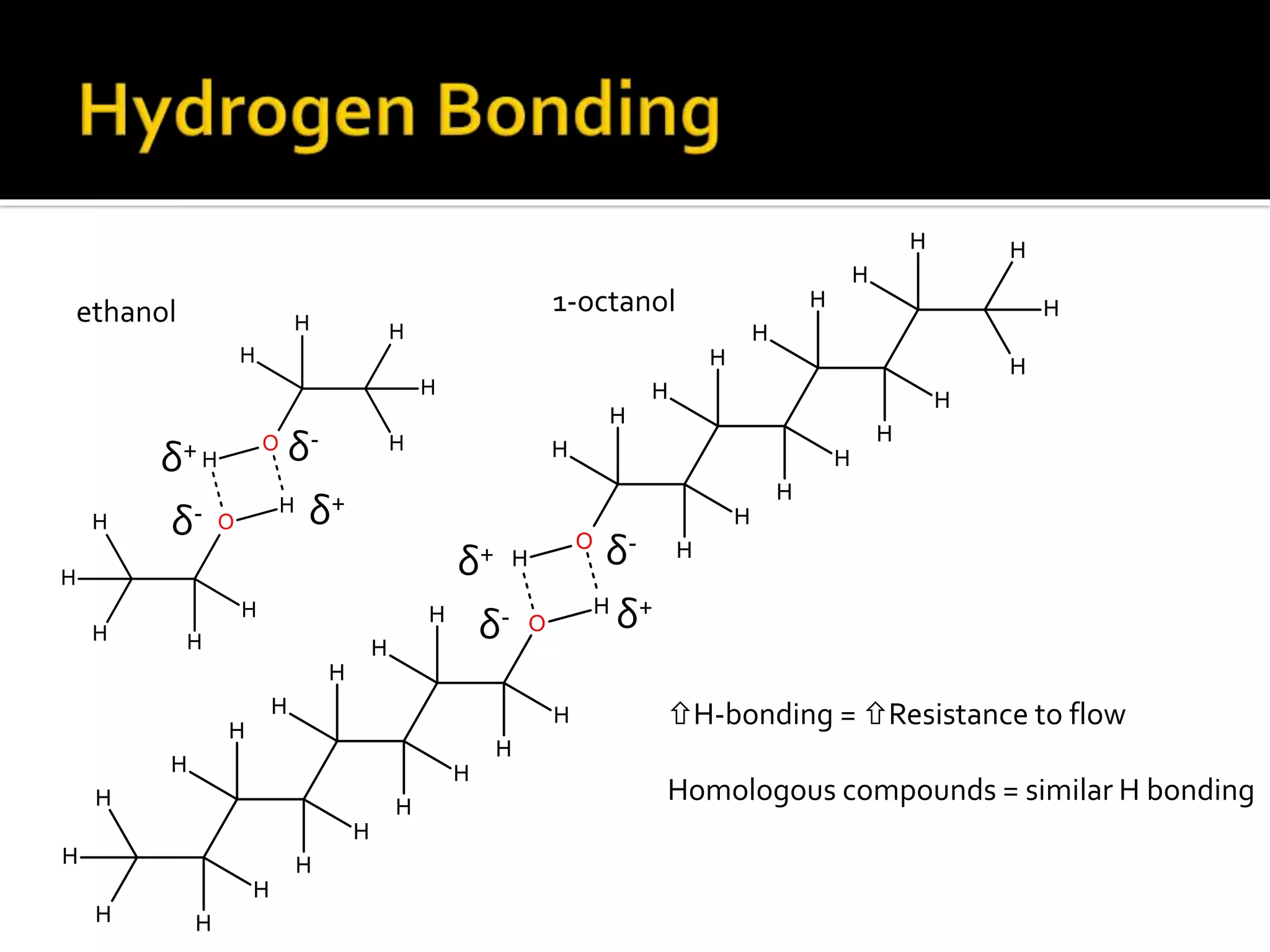
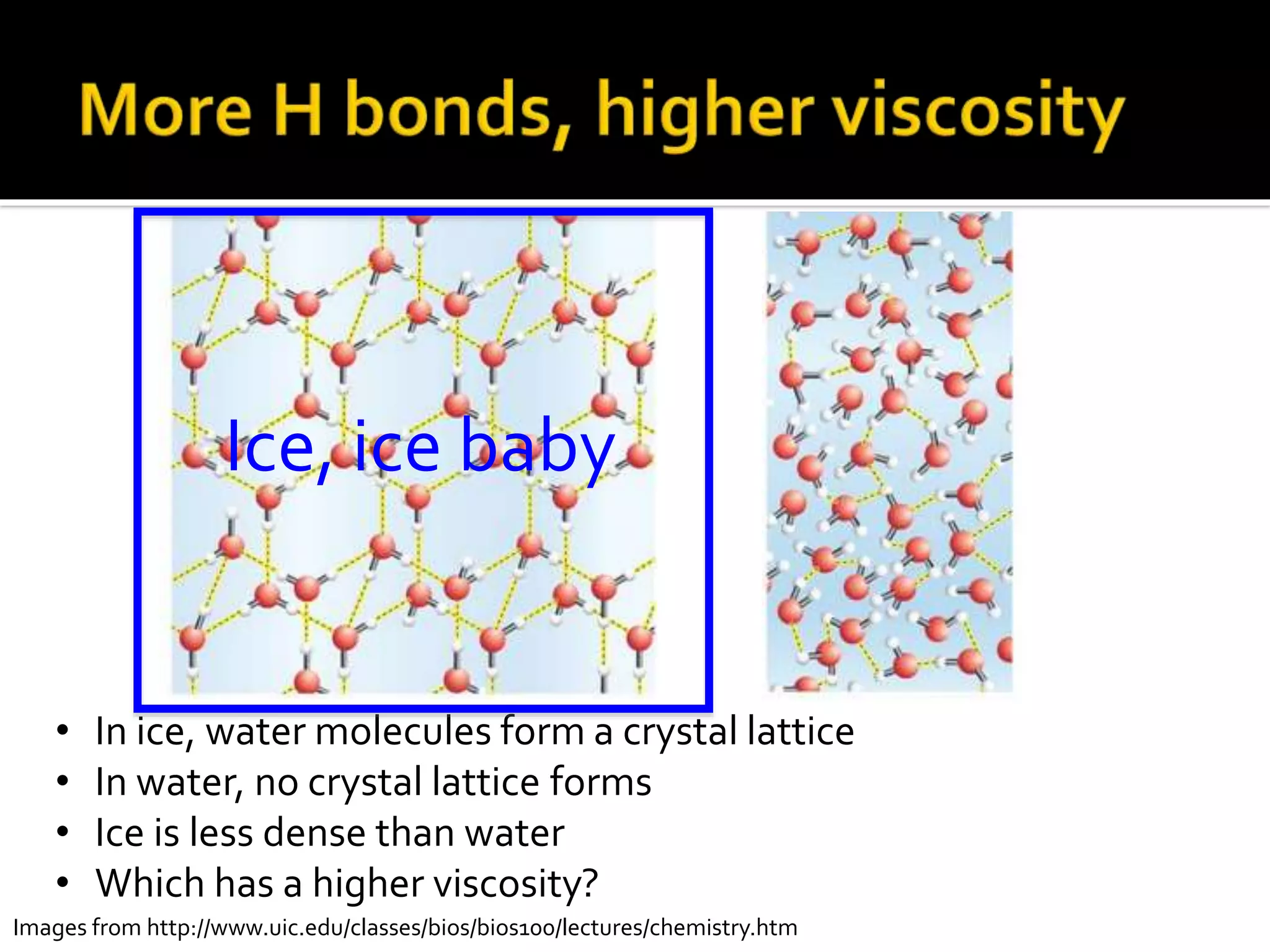
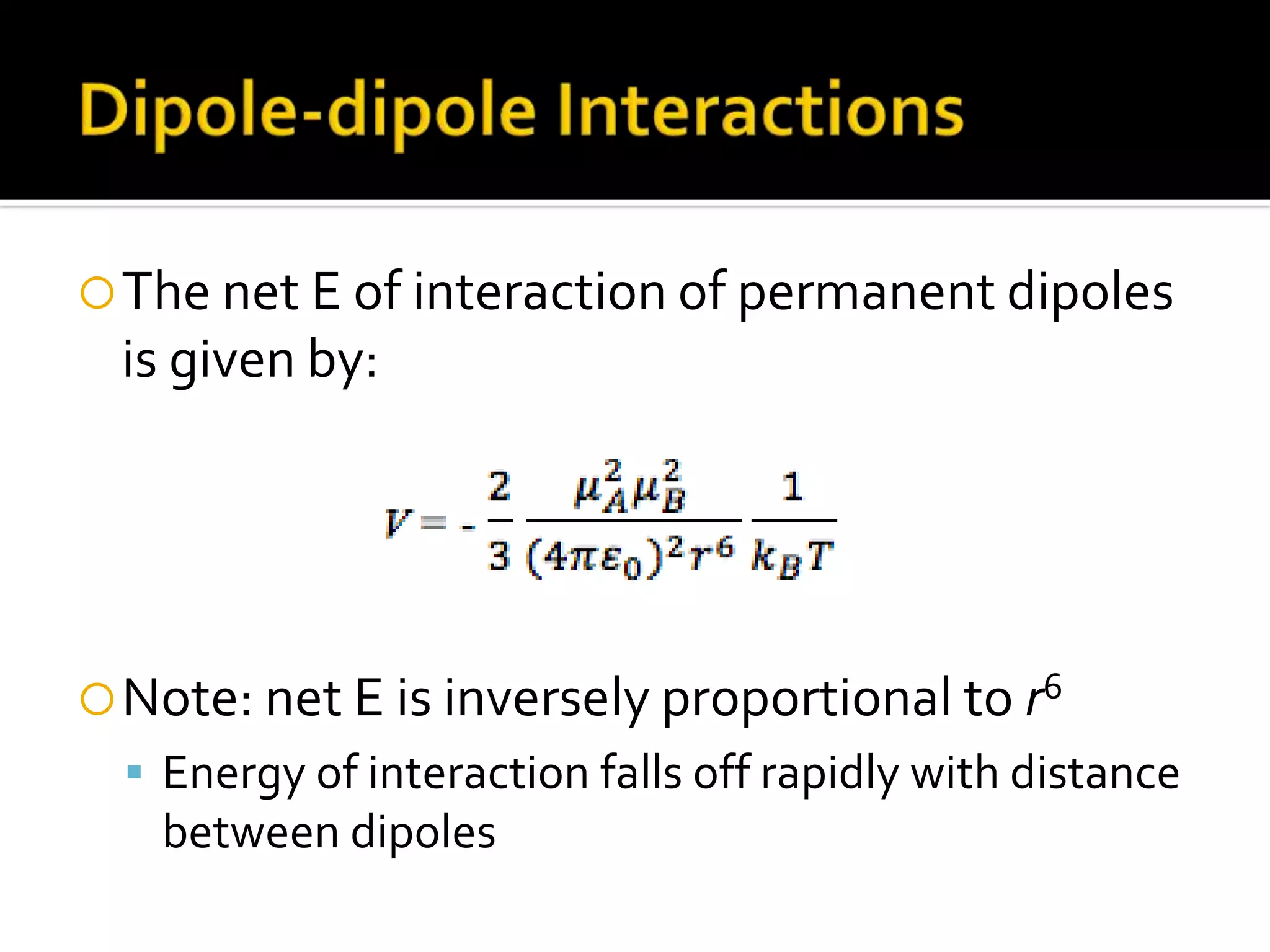
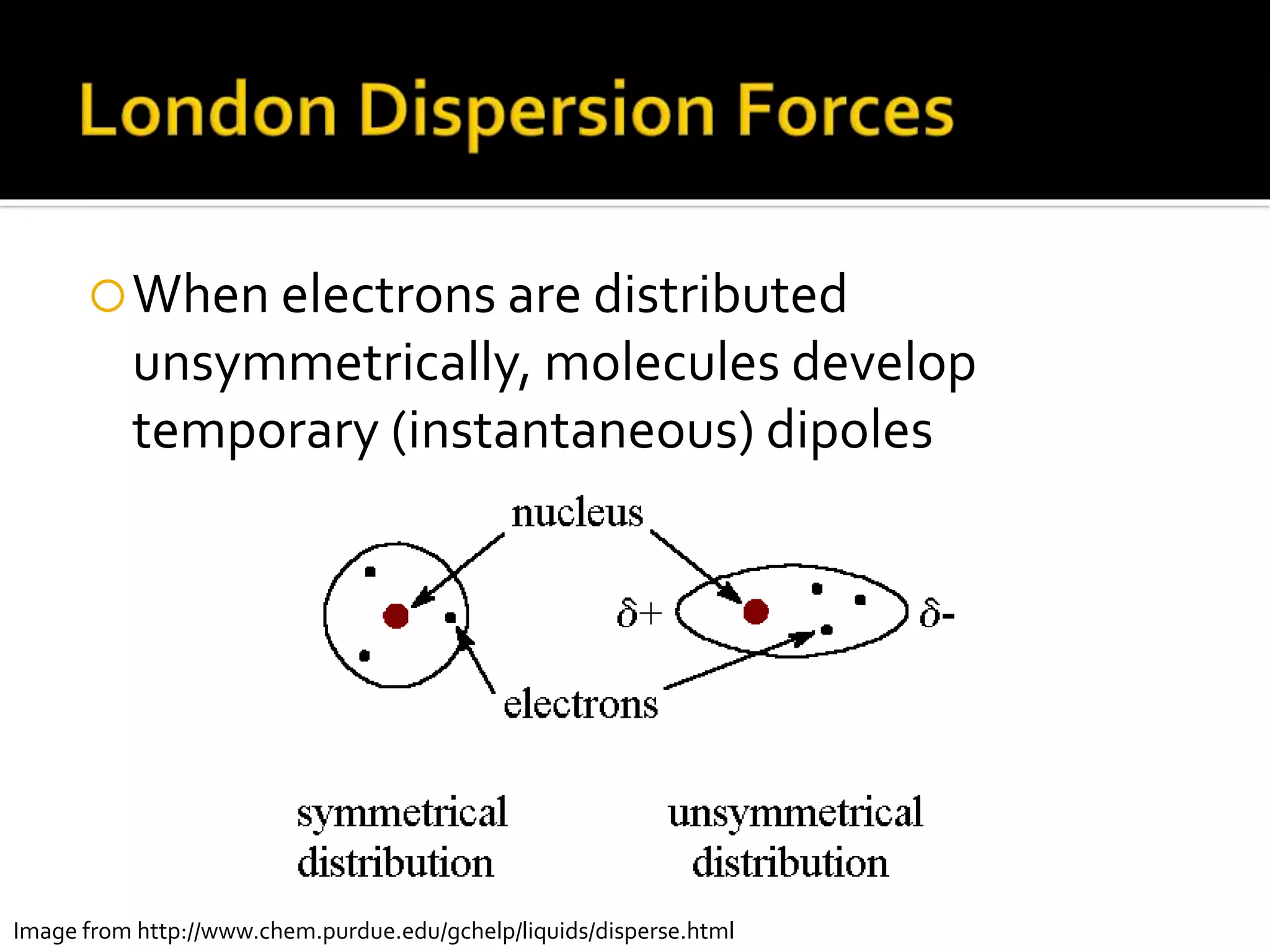
The document discusses the concept of viscosity as the resistance to flow in fluids, comparing the viscosities of various alcohols and their molecular properties. It highlights the role of intermolecular forces such as hydrogen bonding and London dispersion forces in determining viscosity, as well as how molecular weight and density correlate with viscosity. Additionally, it outlines the methodology for measuring viscosity using an Ostwald viscometer and examines experimental results versus literature values for different alcohols.








![Δ%A = (ΔA /A) x 100
Δ%A = (1.3996 x 10-6 mPa cm3 g-1 / 3.499 x 10-3 mPa cm3 g-1) x100
Δ%A= 0.04%
Δ%t = (Δt /t) x 100
Δ%t = (0.1 s / 255.1 s) x100
Δ%t = 0.04%
Δ%η= √[(0.04%)2 + (0.04%)2]
Δ%η= 0.05657%
](https://image.slidesharecdn.com/viscositypresentationppt-140701215236-phpapp01/75/Viscosity-Variations-with-Molecular-Structure-22-2048.jpg)