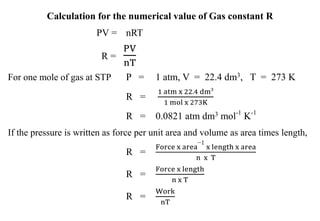
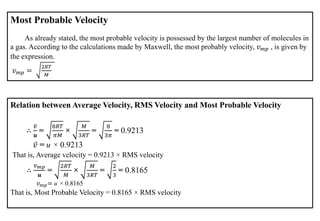
The document discusses the properties of gases. It defines gases as molecules with little attraction between them that are free to move about. It then describes five general characteristics of gases: expansibility, compressibility, diffusibility, pressure, and the effect of heat on gases. The document also discusses gas laws like Boyle's law, Charles' law, Avogadro's law, and the ideal gas law. It derives the kinetic gas equation and explains gas properties in terms of molecular kinetic theory, including the assumptions of kinetic theory and how gas laws can be deduced from the kinetic gas equation.