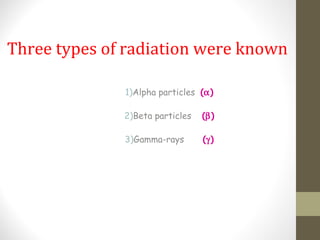
The document provides an overview of radioactivity, including its definition, discovery, types of radiation, and various applications in fields such as cancer treatment and carbon dating. It details the three types of radiation—alpha particles, beta particles, and gamma rays—and discusses radiation controls and the concept of half-life. The document also highlights the advantages and disadvantages of radiation, emphasizing its effectiveness in medical and scientific applications alongside its potential dangers.