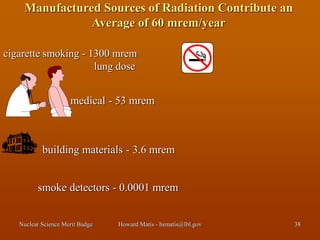
This document discusses various topics related to nuclear science and radiation. It begins by introducing radiation and its relationship to fictional characters gaining superpowers. It then discusses radiation being both dangerous and beneficial. The document goes on to cover topics like atoms, radioactivity, types of radiation, radiation measurement units, radiation health effects, radiation sources, and principles of radiation safety like time, distance and shielding.