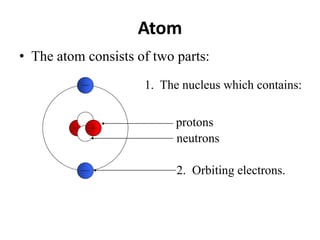
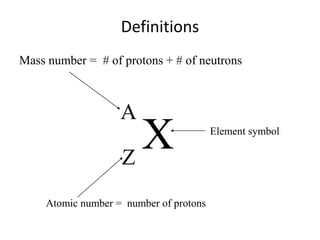
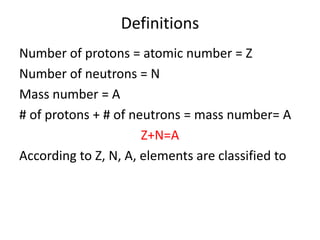
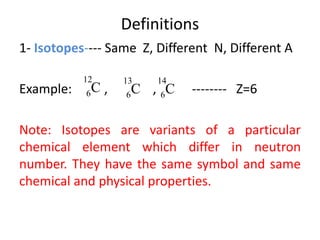
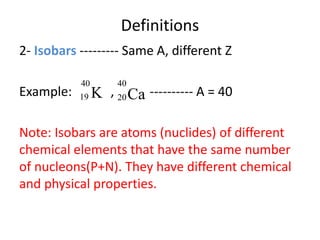
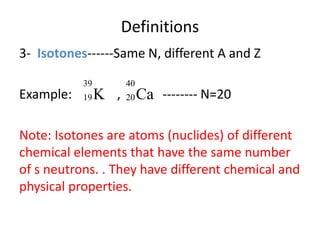
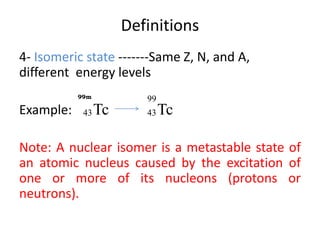
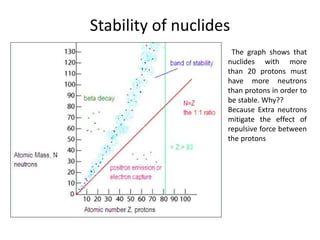
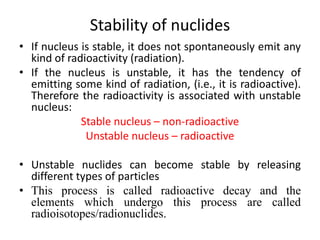
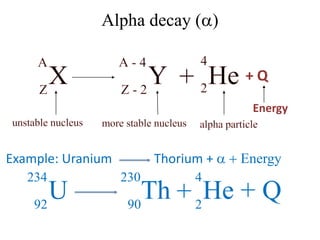
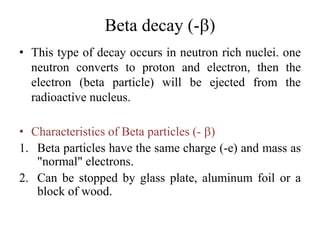
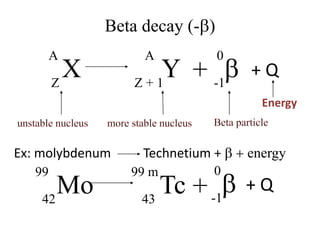
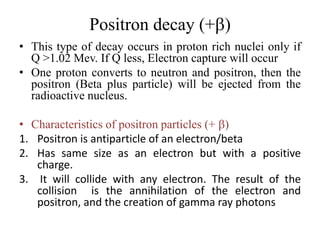
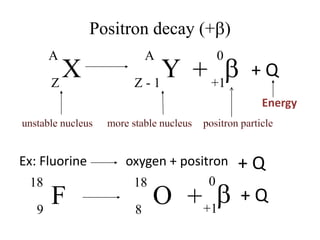
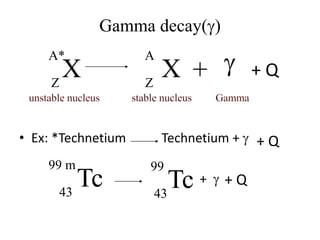
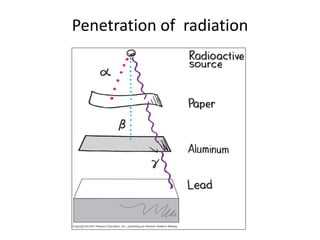
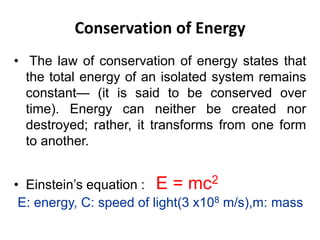
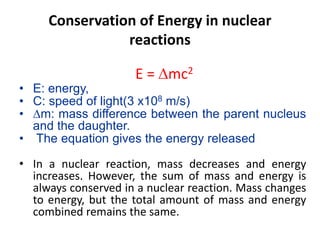
The document discusses radioactive decay and nuclear physics concepts. It describes the discovery of radioactivity by Henri Becquerel and the Curies. It defines key nuclear physics terms like isotopes, isobars, isotones. It explains the different types of radioactive decay including alpha, beta, positron decay and gamma emission. It discusses the penetration and ranges of different types of radiation. Finally, it covers the conservation of energy and mass in nuclear reactions based on Einstein's mass-energy equivalence formula.