This document discusses the discovery and properties of radioactivity. It describes:
- Henri Becquerel's 1896 discovery that uranium salts exposed photographic plates to invisible rays or radiation.
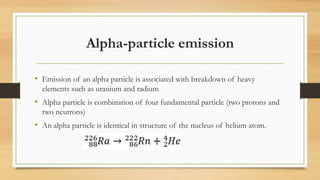
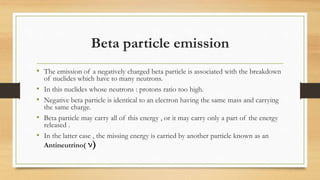
- The three main types of radiation emitted - alpha particles, beta particles, and gamma rays - and their properties like penetrating power and interaction with matter.
- How radioactive decay occurs via emission of these particles or photons from atomic nuclei, and the relationship between decay constant and half-life of radioactive substances.