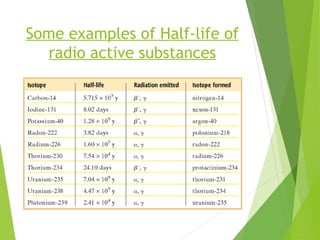
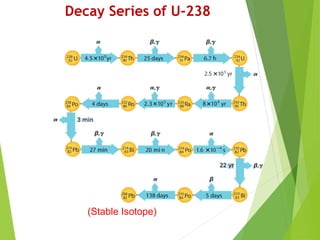
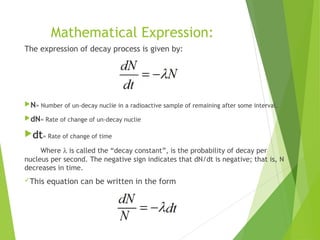
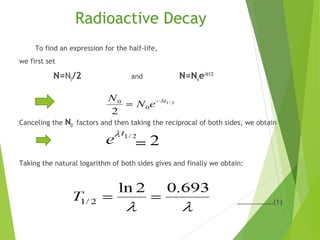
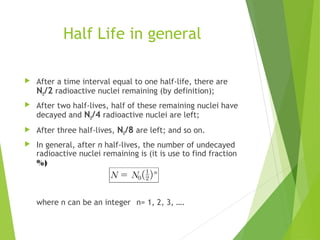
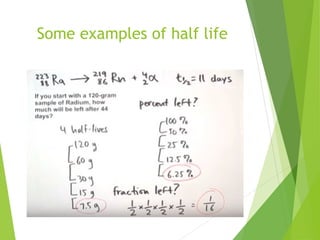
The document discusses the concept of half-life, which is defined as the time it takes for half of the nuclei in a radioactive sample to decay. Each radioactive isotope has its own characteristic half-life that can range from fractions of a second to billions of years. The number of undecayed nuclei decreases exponentially over time according to the radioactive decay equation. Examples of half-lives for some isotopes like uranium-238 and carbon-14 are provided to illustrate the range and applications of half-life.