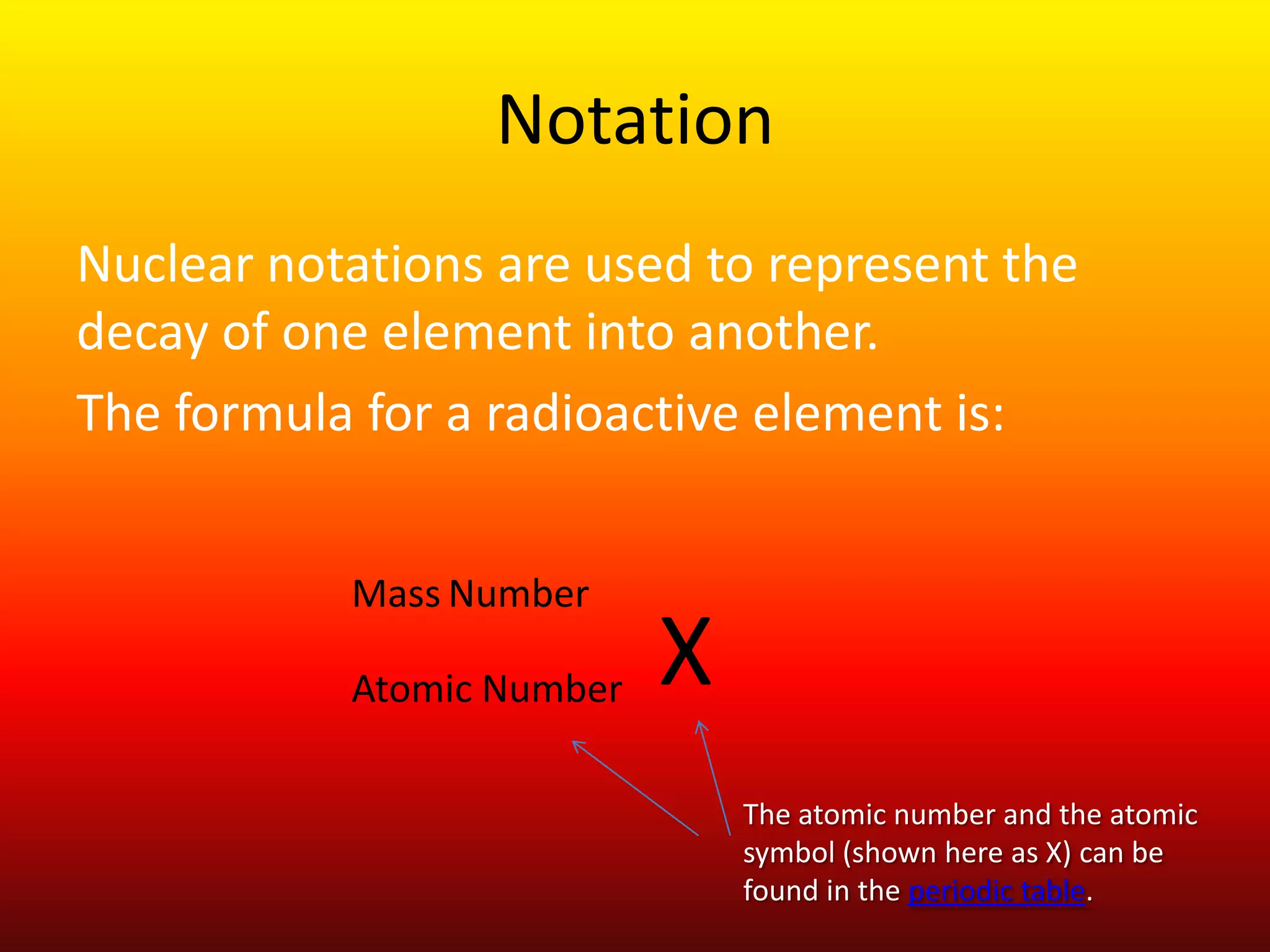
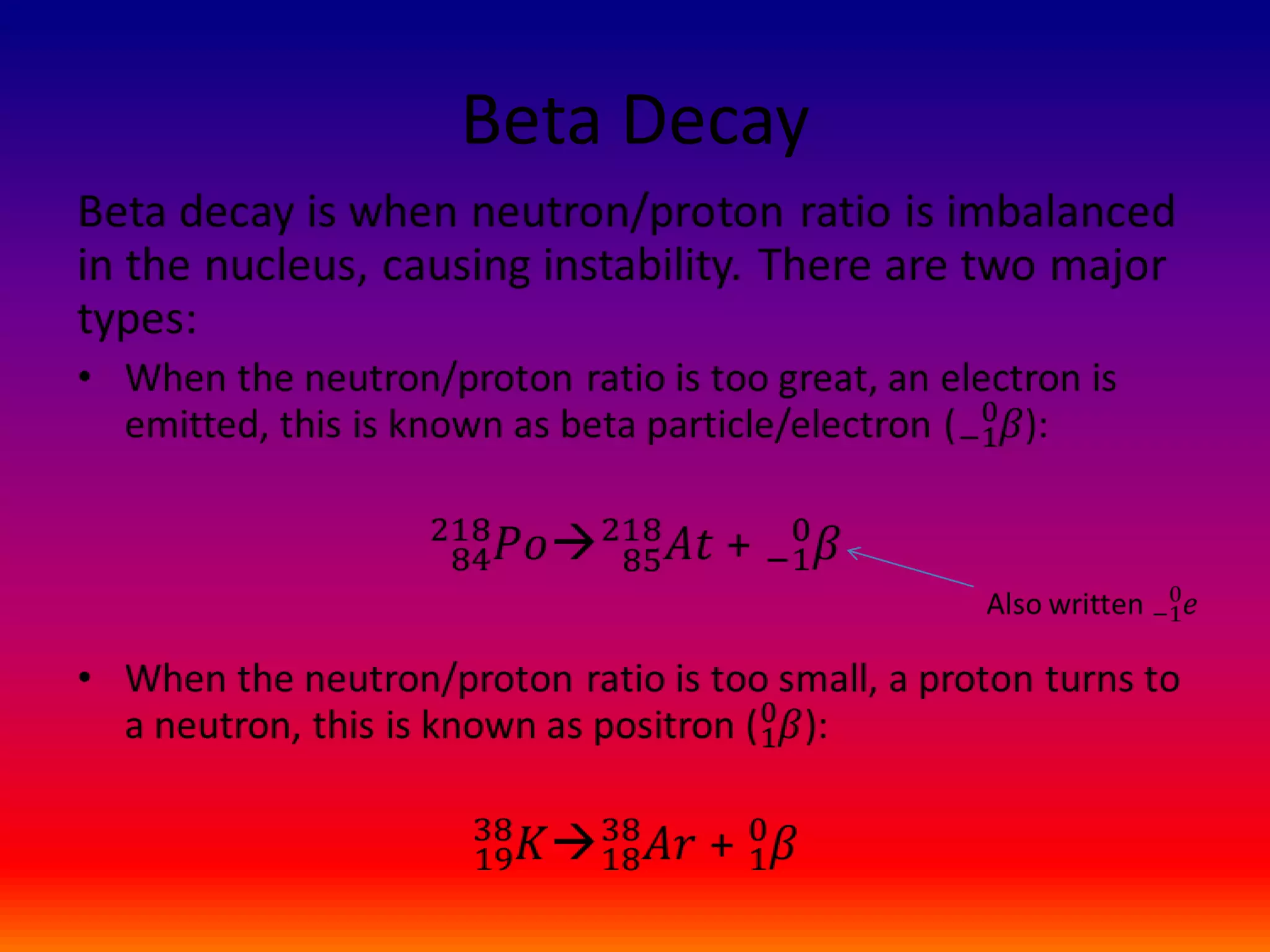
The document discusses radioactive decay and nuclear chemistry. It defines radioactive decay as the spontaneous disintegration of a radionuclide accompanied by the emission of ionizing radiation such as alpha or beta particles or gamma rays. It notes that radioactive decay changes an unstable element into a stable element. The document also discusses alpha decay, beta decay, gamma decay, and provides examples of nuclear notation used to represent radioactive decay.