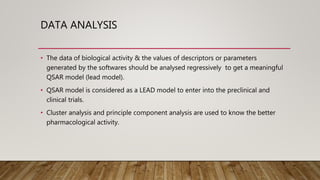
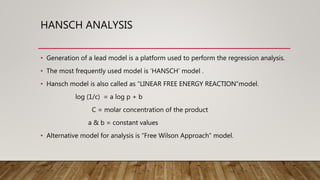
QSAR (Quantitative Structure Activity Relationship) is a computational method that correlates chemical structures or properties of compounds to their biological activity. It transforms chemical structures into numerical descriptors for properties like solubility or log P (partition coefficient). QSAR models then use these descriptors in mathematical formulas to quantitatively predict biological activity based on structural or physicochemical properties. Key parameters used in QSAR include log P (measure of lipophilicity), Hammett constants (measure electron donating/withdrawing ability), and Taft steric parameters (measure steric effects on size and shape). Hansch analysis and linear free energy models are commonly used methods to perform regression analysis of biological activity data and generate predictive QSAR models.










![• For example in octanol-water system is
log P oct/wat = log [solute] unionized
[solute] unionised
log P = log {organic phase}
log {acqueous phase}
log P = log
octanol
water
Unionised compound
octanol
water](https://image.slidesharecdn.com/qsar-200416070325/85/QSAR-11-320.jpg)