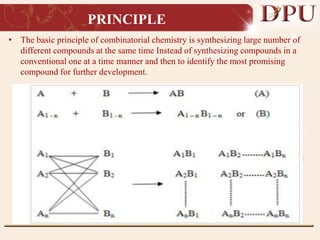
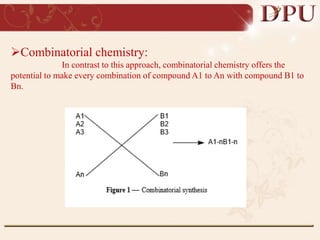
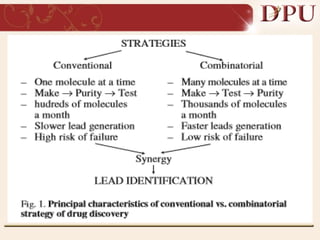
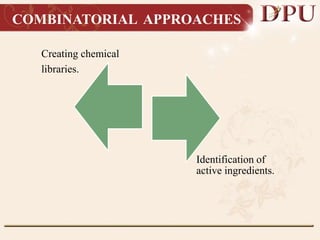
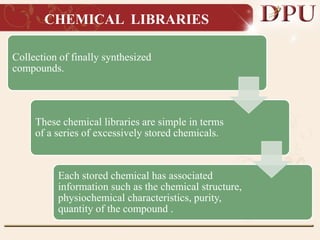
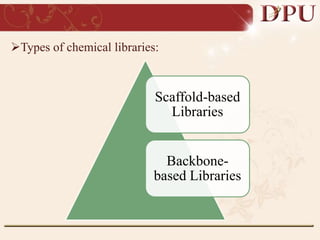
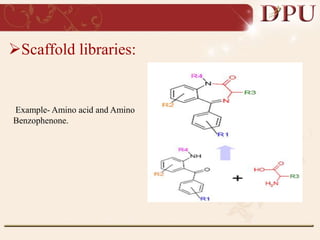
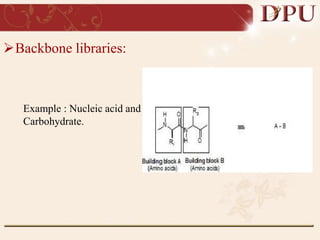
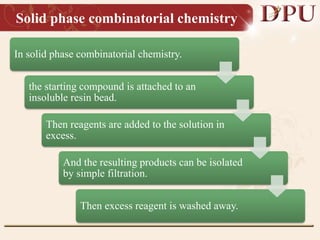
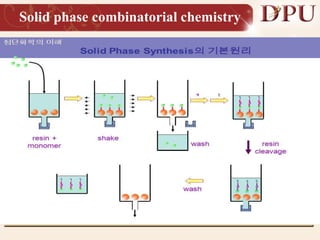
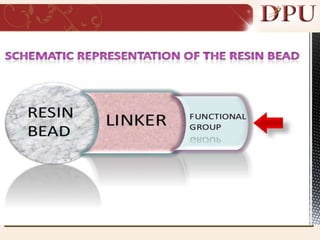
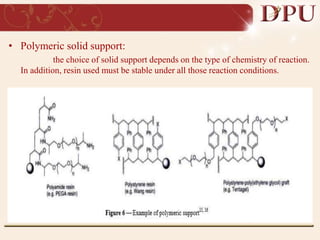
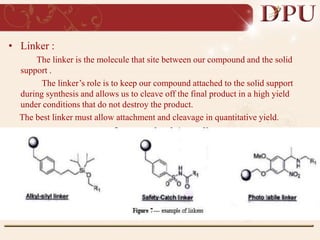
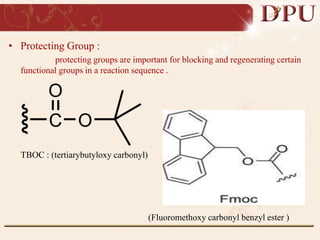
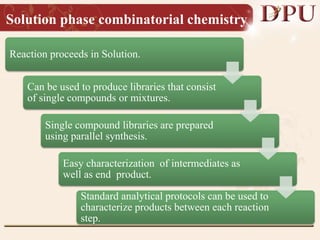
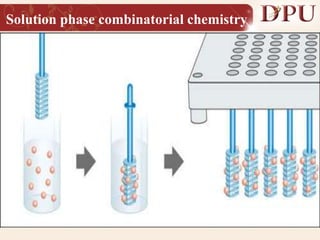
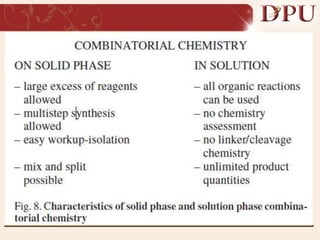
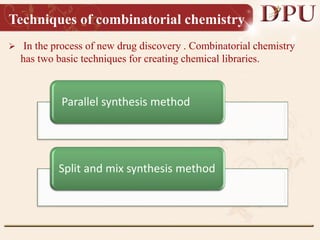
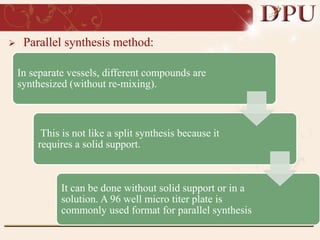
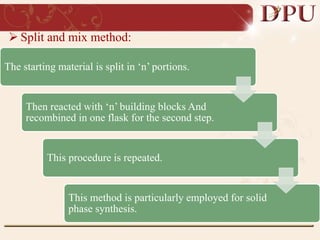
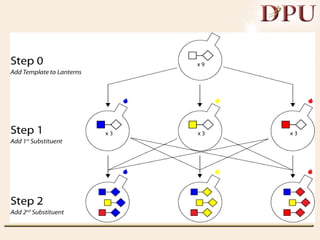
Combinatorial chemistry allows for the rapid synthesis of large libraries of compounds. It works by synthesizing many structures in parallel rather than one at a time. There are two main approaches: solid phase synthesis which attaches compounds to resin beads to isolate products, and solution phase which synthesizes in solvent. The libraries can be screened to identify active compounds more efficiently than traditional methods. This technique has increased the success of drug discovery by allowing testing of more structures at once.