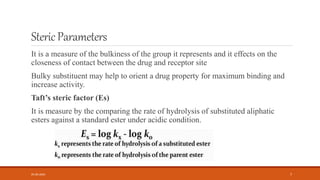
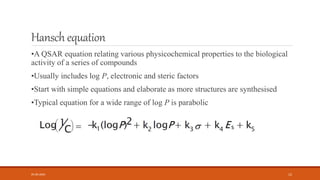
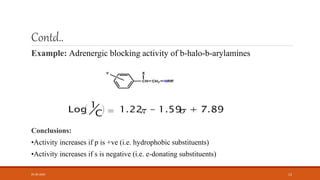
This document discusses various quantitative structure-activity relationship (QSAR) methods including physicochemical parameters, Hansch analysis, and Free-Wilson analysis to correlate biological activity of compounds to their chemical structure. It describes measuring hydrophobicity using partition coefficients and electronic effects using Hammett constants. Hansch analysis relates biological activity to multiple physicochemical properties through linear or parabolic equations. Free-Wilson analysis quantifies the contribution of individual substituents to activity without needing physicochemical constants. Both approaches aim to predict new compounds' activities based on structural attributes.