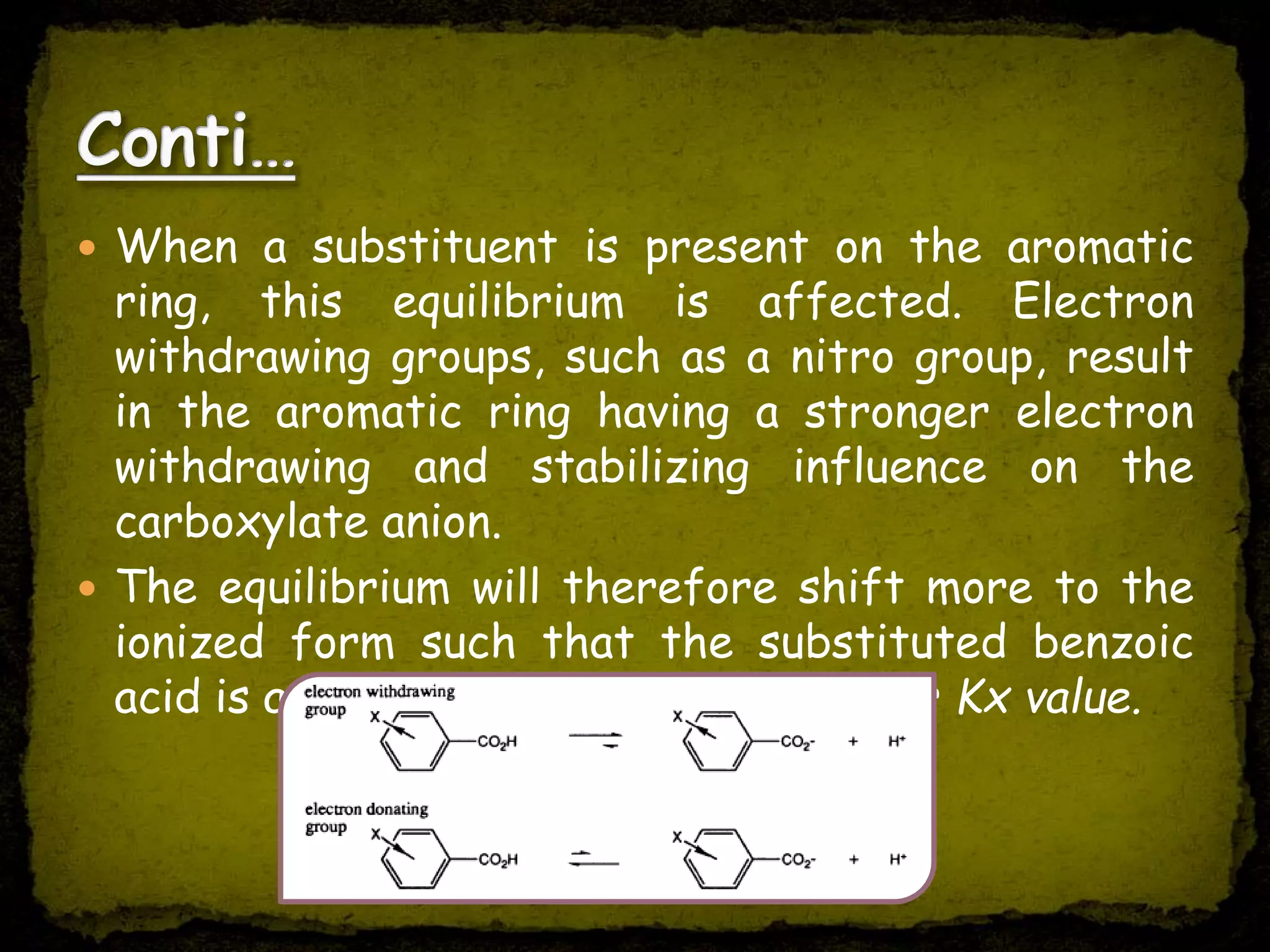
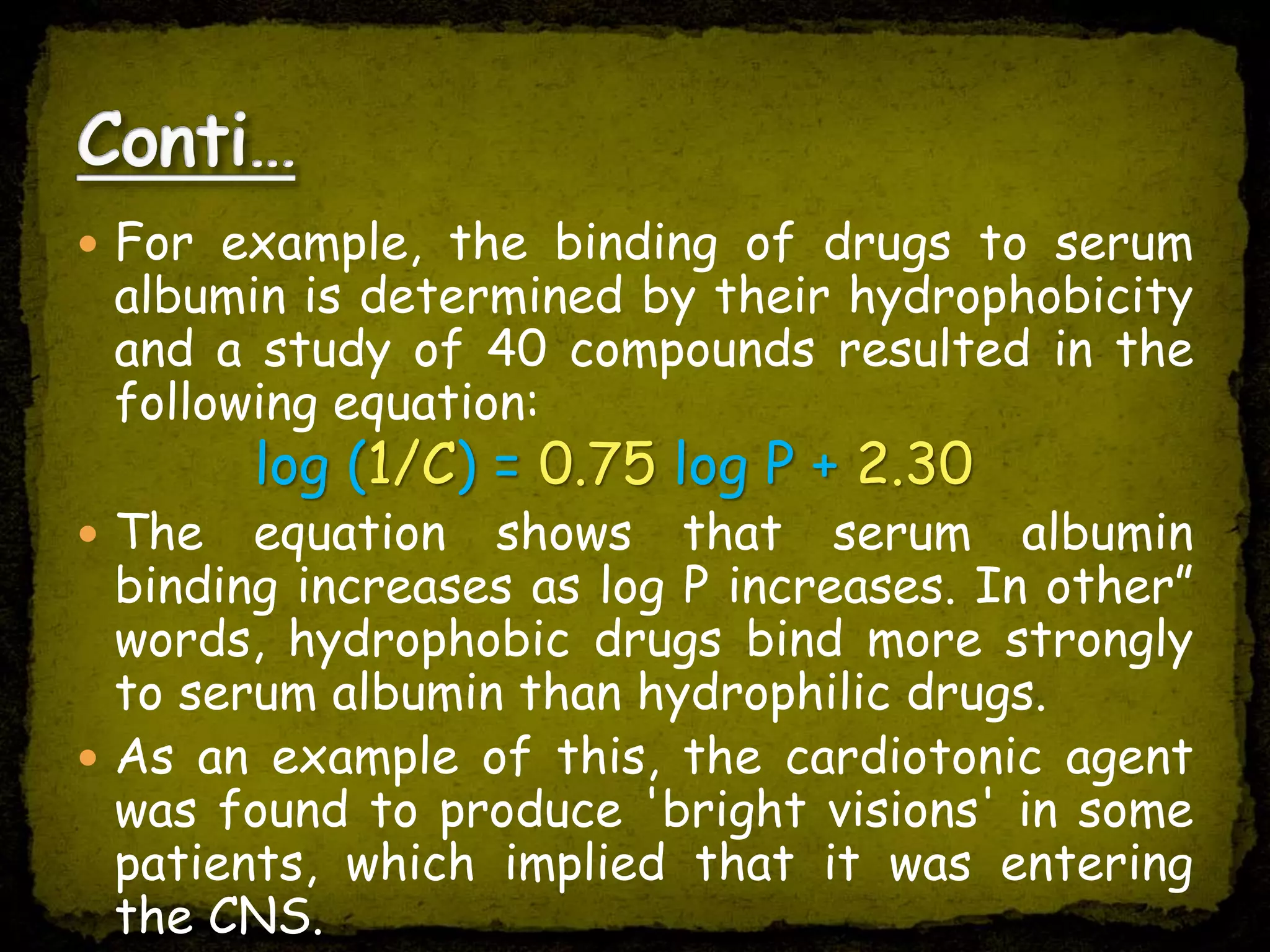
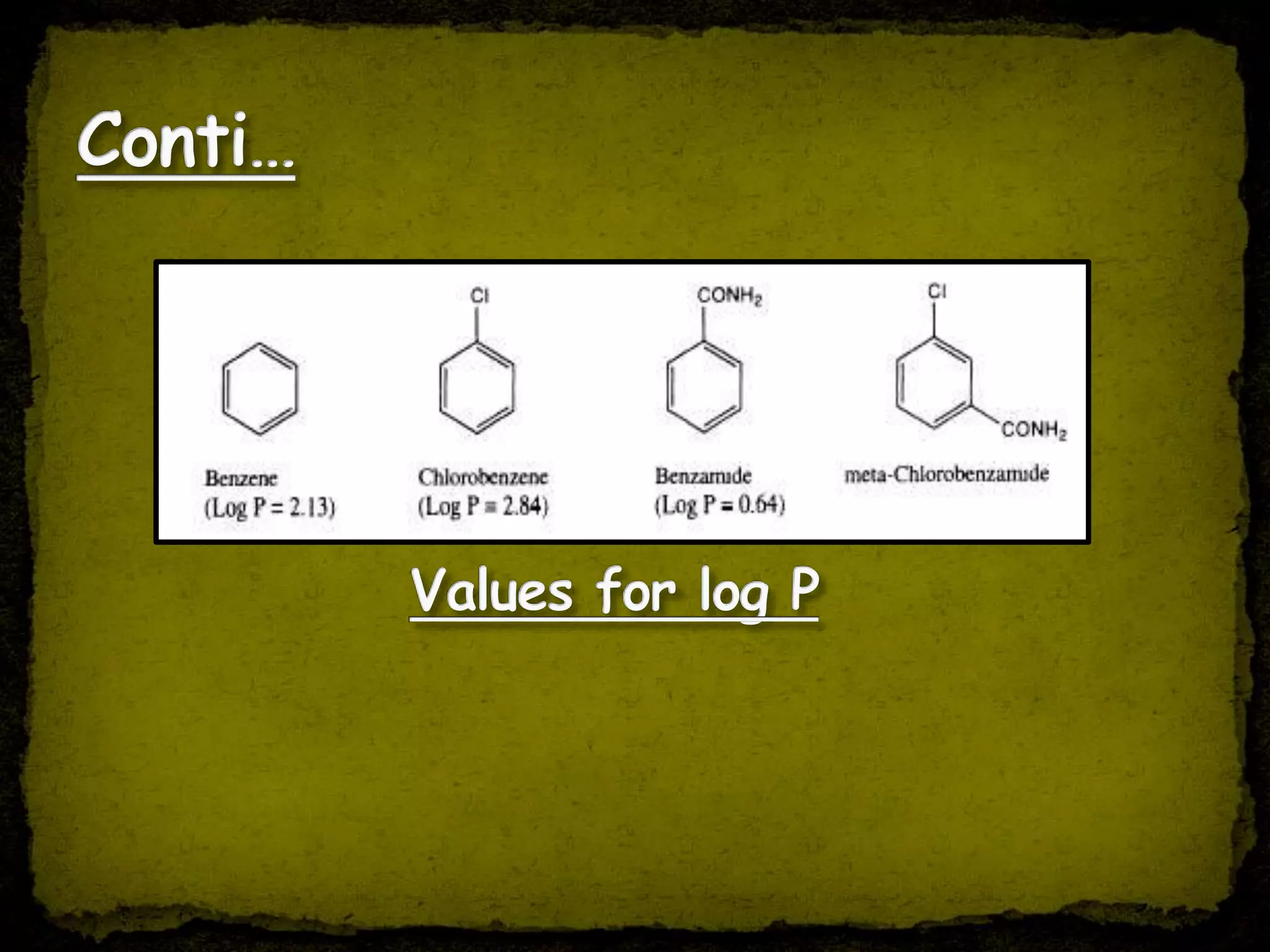
1. Quantitative Structure Activity Relationship (QSAR) uses mathematical models to correlate chemical descriptors of molecules to their biological activity.
2. Descriptors include physicochemical properties like hydrophobicity which can be measured experimentally.
3. QSAR allows medicinal chemists to predict activity of novel analogues and guide synthesis toward more active compounds.












![ Benzoic acid is a weak acid and only partially
ionizes in water. An equilibrium is set up between
the ionized and non-ionized forms.
Dissociation constant (KH)=[PhCO0¯]/[PhCO0H]
Ionization of benzoic acid](https://image.slidesharecdn.com/ligandbaseddrug-design-170419073028/75/Ligand-based-drug-design-15-2048.jpg)