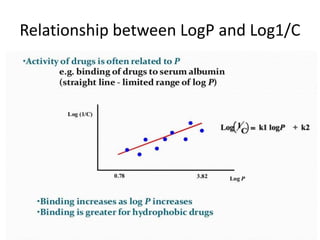
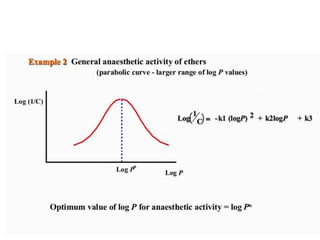
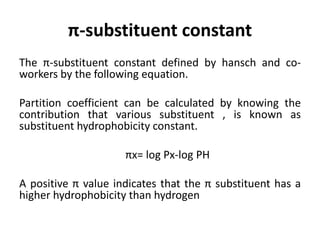
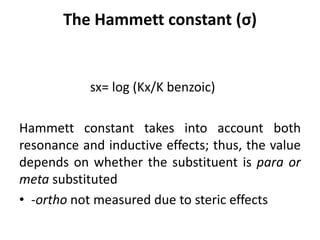
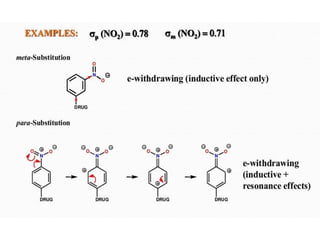
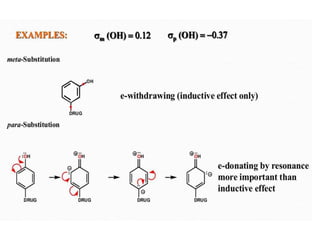
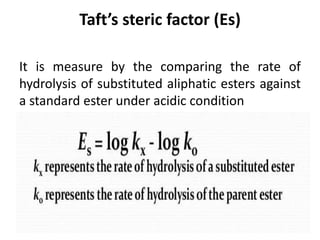
This document discusses QSAR (quantitative structure-activity relationship), which is a mathematical model that relates biological activity to physicochemical properties of molecules. QSAR can be used to predict activity of new compounds. Key parameters used in QSAR include hydrophobicity (measured by partition coefficient), electronic effects (Hammett constant), and steric effects (Taft's steric factor). These parameters influence drug absorption, distribution, and interactions with receptors. QSAR models take the form of biological activity = function(parameters) to develop relationships between activity and molecular properties.