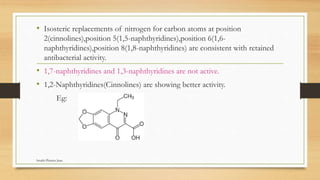
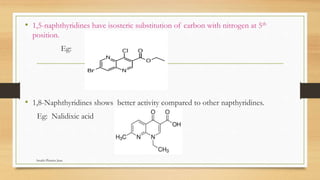
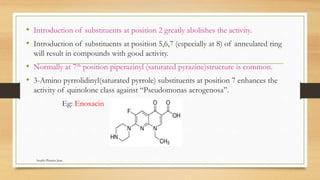
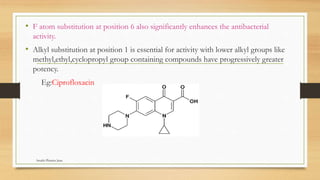
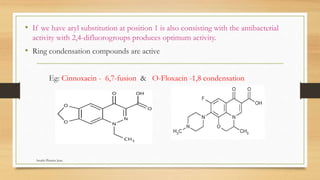
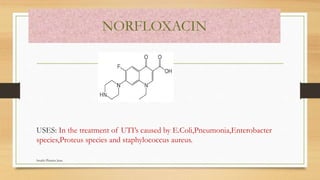
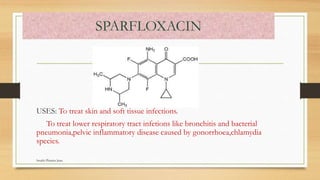
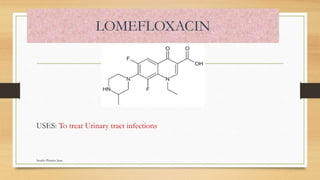
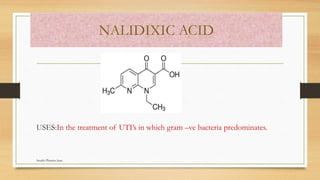
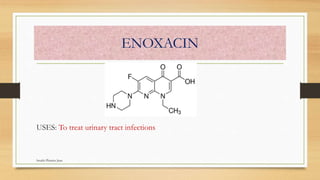
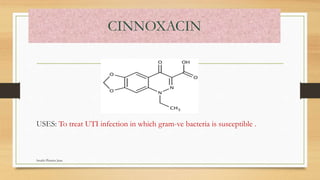
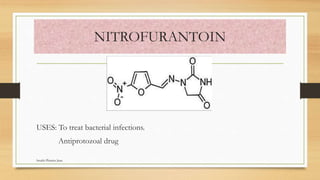
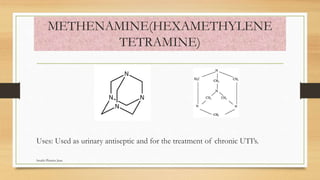
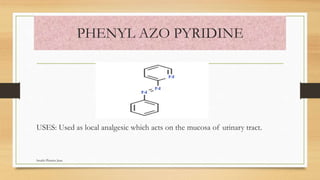
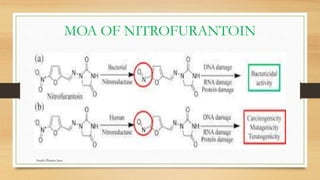
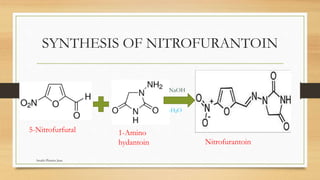
This document discusses urinary tract anti-infective agents. It classifies these agents based on their chemical structure into quinolone derivatives, nitrofuran derivatives, methenamine and its salts, and urinary analgesics. It provides details on various quinolone derivatives like norfloxacin, ciprofloxacin, and nalidixic acid. It describes the structure-activity relationships and mechanisms of action of quinolone derivatives and nitrofurantoin. It lists the uses of various urinary tract anti-infective agents in treating infections like UTIs, pneumonia, and pelvic inflammatory disease.