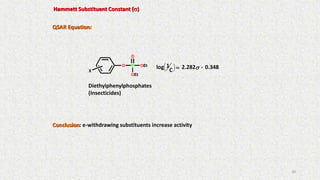
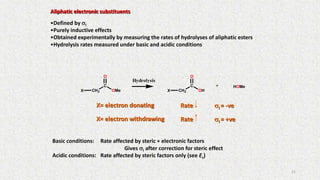
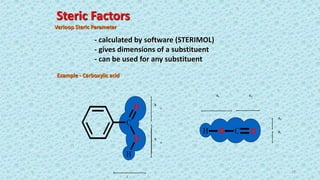
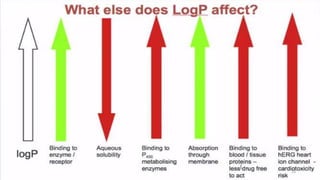
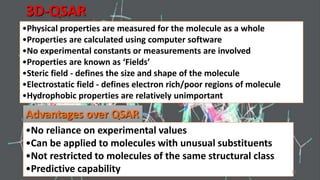
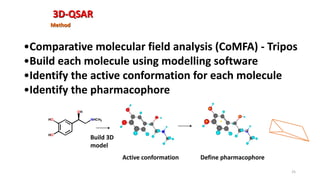
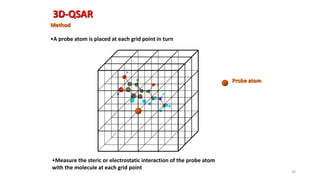
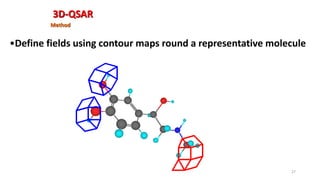
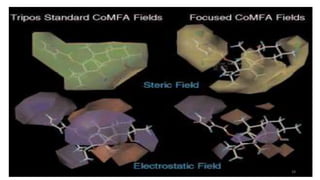
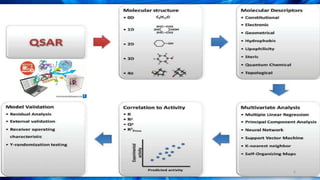
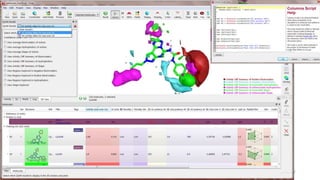
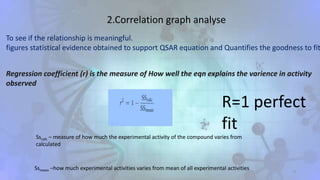
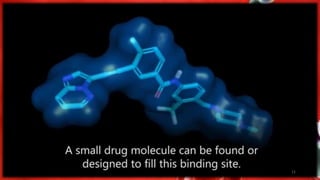
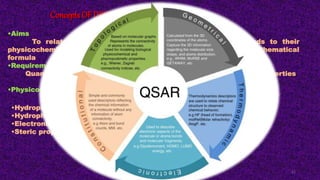
The document discusses the quantitative structure-activity relationship (QSAR) approach in drug design, highlighting the importance of identifying and quantifying the physicochemical properties' effects on drug biological activity. It explores the use of linear regression analysis to predict drug interactions and the role of electronic and steric factors in determining compound activity. Additionally, the document introduces 3D-QSAR methods that analyze molecular properties without relying on experimental data, enhancing predictive capabilities for drug design.




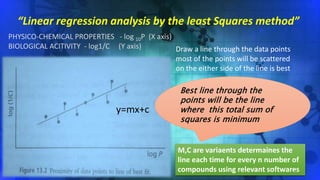

![Hydrophobicity of the Molecule
Partition Coefficient P = [Drug in octanol]
[Drug in water]
High P High hydrophobicity
•Activity of drugs is often related to P
e.g. binding of drugs to serum albumin
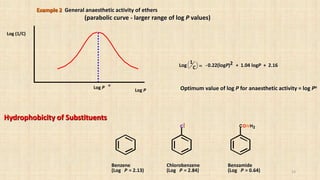
(straight line - limited range of log P)
Log (1/C)
Log P
. .
.
.. .
. ..
0.78 3.82
Log 1
C
= 0.75 logP + 2.30
•Binding increases as log P increases
•Binding is greater for hydrophobic drugs
13](https://image.slidesharecdn.com/quantitativestructureactivityrelationship-180309085920/85/Quantitative-structure-activity-relationship-QSAR-13-320.jpg)

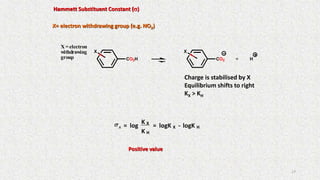
![Electronic Effects
Hammett Substituent Constant (s)
Notes:
•The constant (s) is a measure of the e-withdrawing or e-donating influence of
substituents
•It can be measured experimentally and tabulated
(e.g. s for aromatic substituents is measured by comparing the
dissociation constants of substituted benzoic acids with benzoic acid)
X=H K H = Dissociation constant= [PhCO 2-]
[PhCO 2H]
+CO2H CO2 H
X X
16](https://image.slidesharecdn.com/quantitativestructureactivityrelationship-180309085920/85/Quantitative-structure-activity-relationship-QSAR-16-320.jpg)