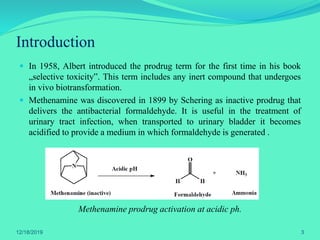
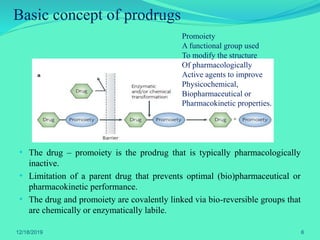
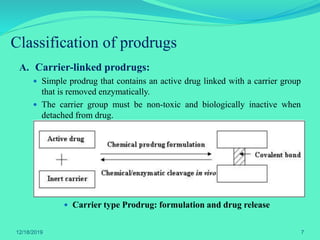
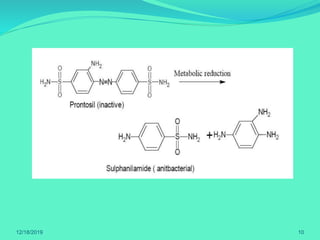
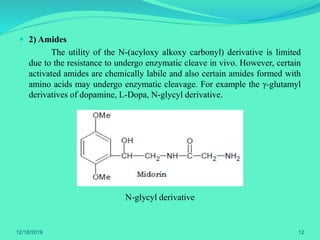
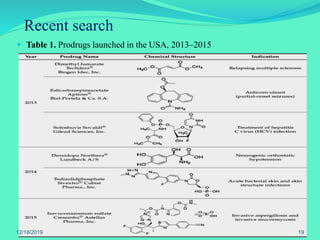
The document discusses prodrugs, which are pharmacological substances administered in inactive forms that metabolize into active drugs in the body. It covers the definition, design, objectives, classifications, and applications of prodrugs, highlighting their importance in improving drug delivery and pharmacokinetics. Additionally, it provides examples and recent developments in prodrug research.