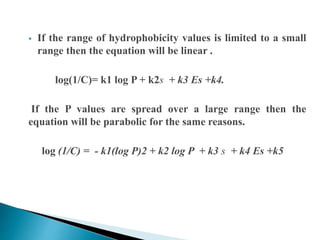
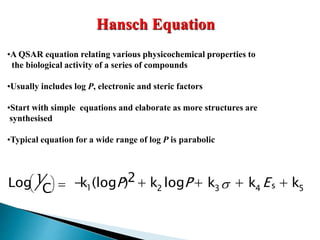
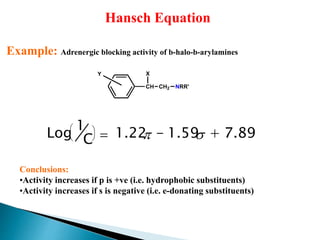
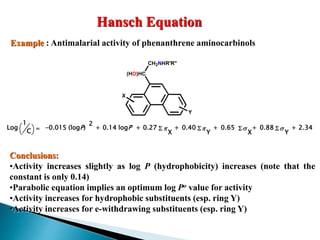
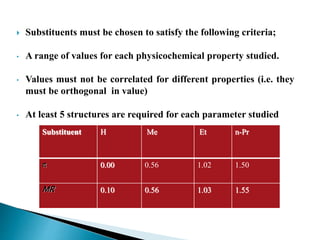
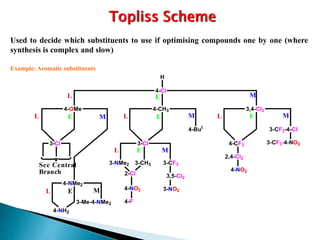
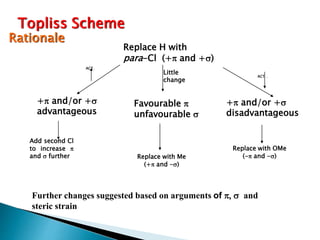
This document provides an overview of quantitative structure-activity relationship (QSAR) modeling techniques including Hansch analysis, Free-Wilson analysis, and Topliss schemes. It discusses how QSAR relates the biological activity of drugs to their physicochemical properties through equations. Specifically, it explains that Hansch equations relate activity to hydrophobicity, electronic effects, and steric factors. Examples of Hansch equations are provided. The Free-Wilson approach derives equations based on the presence or absence of substituents. Topliss schemes provide a methodical approach to substituent selection for optimization.