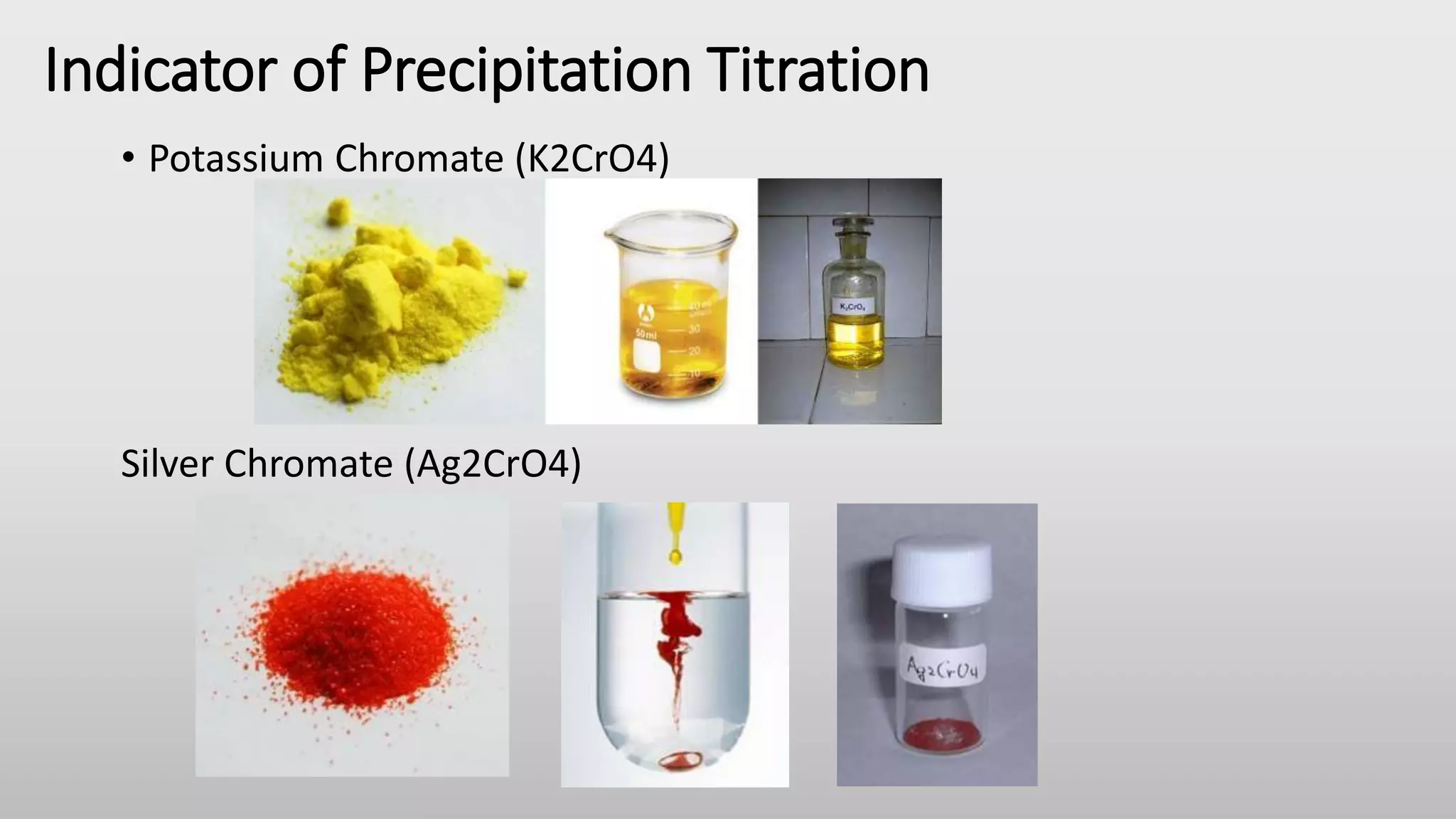
Precipitation titration involves the formation of an insoluble salt precipitate between the titrant and analyte. This titration continues until all of the analyte is consumed. Some common precipitation titrations include the titration of silver nitrate with halide ions like chloride. There are several methods for precipitation titration including Mohr's method, Volhard's method, and Fajan's method which use different indicators to detect the endpoint. Precipitation titration has limitations in detecting the endpoint and potential co-precipitation, but it can be used to quantitatively analyze ions like bromide, chloride, carbonate, iodide, sulfide, and thiocyanate.