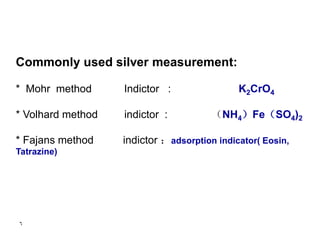
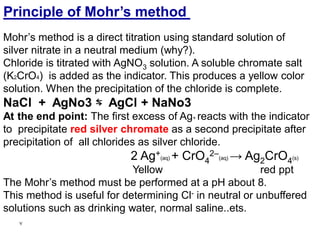
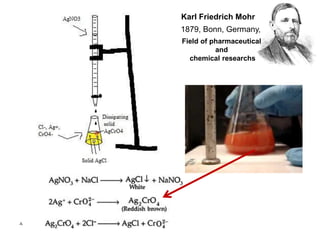
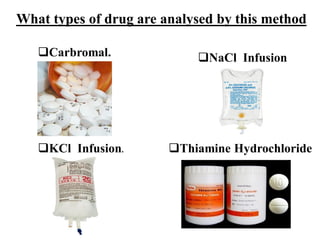
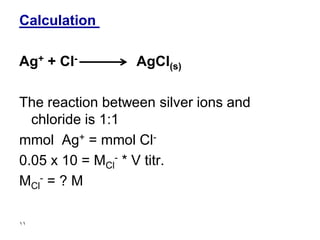
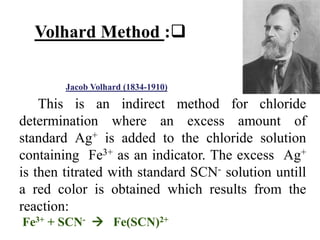
This document discusses precipitation titrations, specifically Mohr's method for determining chloride concentration. Mohr's method involves titrating a chloride sample with silver nitrate solution until a red silver chromate precipitate forms, indicating the endpoint. The reaction between silver ions and chloride ions forms an insoluble silver chloride precipitate. Potassium chromate is used as the indicator which changes color upon reaction with excess silver ions after all chloride is precipitated. Mohr's method provides an accurate means of determining chloride concentration in neutral or unbuffered solutions like drinking water or saline solutions.