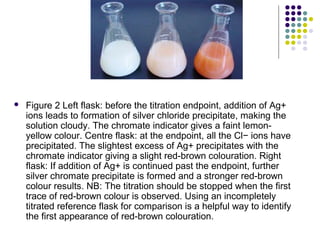
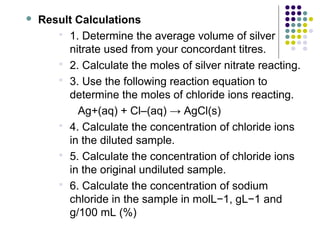
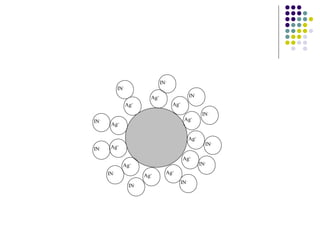
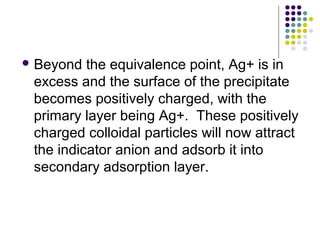
This document describes the precipitation method for determining the chloride ion concentration of a solution by titration with silver nitrate. Silver nitrate is added until all chloride ions are precipitated as silver chloride. Additional silver ions then react with potassium chromate indicator to form a red-brown silver chromate precipitate, signaling the endpoint. The method can be used to analyze water samples. It involves titrating aliquots of the sample with a standardized silver nitrate solution until concordant results are obtained.