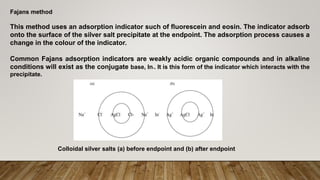
Precipitation titrations involve the titration of an analyte with a reagent to form an insoluble precipitate. The most common precipitation reaction used is between silver ions and chloride, bromide, iodide or thiocyanate ions. This type of titration is called an argentometric titration. Common methods for argentometric titrations include Mohr's method, which uses potassium chromate as an indicator, and Fajan's method, which uses an adsorption indicator that changes color when it adsorbs onto the precipitate surface at the endpoint.