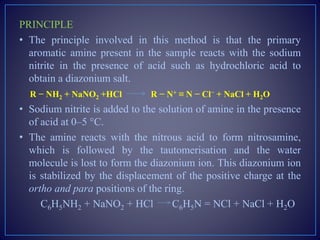
The document provides information about diazotization titrations. It discusses the principle, theory, procedure, end point detection, factors affecting, applications, and advantages/disadvantages of diazotization titrations. The key points are:
- Diazotization titrations involve the reaction of a primary aromatic amine with sodium nitrite in acidic medium to form a diazonium salt, which is then titrated.
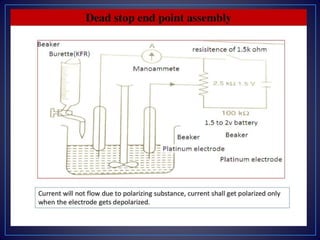
- The end point is detected using an external indicator like starch iodide paper or electrochemically.
- Factors like acid concentration, temperature, and reaction time must be controlled.
- It can be used to determine drugs and compounds containing
















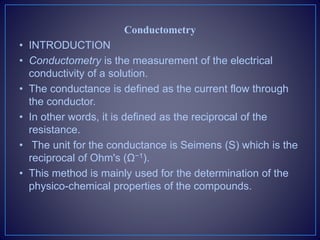




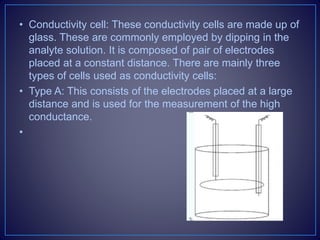
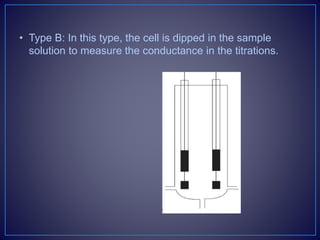
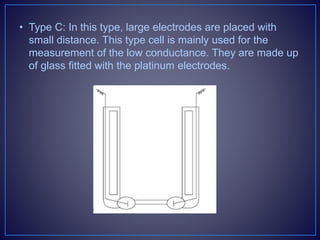


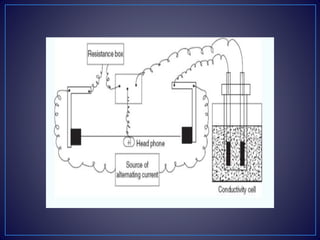

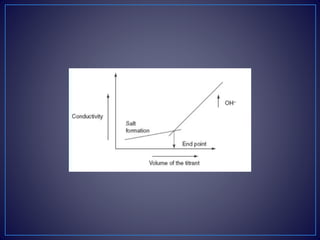
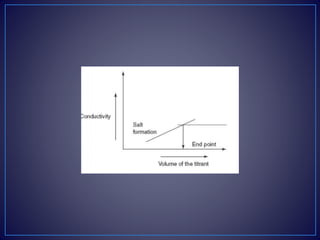
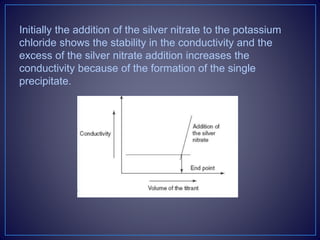
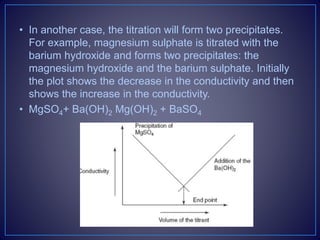
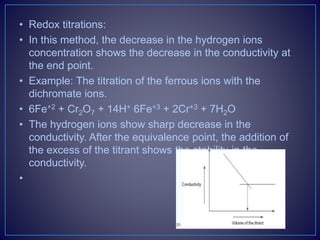
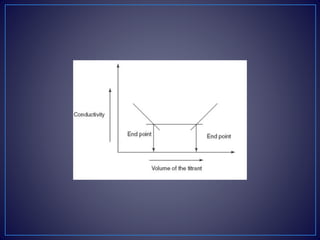
![• Types of the Conductometric Titrations
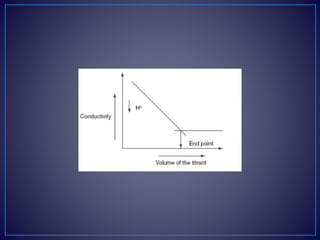
• Acid–base titrations: In this method, the conductance of
the hydrogen ions and hydroxyl ions are compared with
the conductivity of the sample solution.
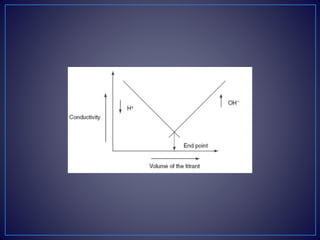
• Strong acid with a strong base:
• For example, take the titration of the HCl with NaOH.
• [H+Cl−] + [Na+OH−] [Na+Cl−] + H2O
• The initial conductivity of the HCl solution is high because of the
protons from the dissociation of the acid. Then titrating with NaOH
dissociates into Na+ and OH−. This hydroxyl ion reacts with the H+
ions to form the water. This shows the decrease in the
conductivity. After completion of the reaction, the excess addition
of the NaOH shows the increase in the conductivity. The plot
between the conductivity and the volume of the titrant shows the
V-shaped curve.](https://image.slidesharecdn.com/diazotizationtitrations-210501133140/85/Diazotization-titrations-32-320.jpg)