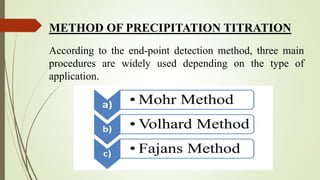
The document outlines the principles and methods of precipitation titration, including Mohr's method, Fajan's method, and Volhard's method, commonly used in pharmaceutical chemistry to determine ion concentrations. It describes how precipitation reactions occur based on the solubility of the analyte and titrant, and details specific procedures and indicators used in each method. The document serves as an educational resource for 1st-year pharmacy students presented by Ms. Shweta Singh.



![MOHR’S METHOD
Karl Friedrich Mohr was the scientist who introduced this method.
Titrant - 0.1 N AgNO3 (Silver nitrate)
Analyte – 0.1 N NaCl (Sodium chloride)
Indicator – 5% K2CrO4 (Potassium chromate)
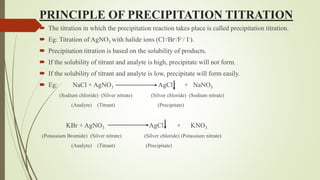
Principle:
NaCl + AgNO3 AgCl + NaNO3 Ksp[AgCl]= 1.2X 10-10
K2CrO4 + AgNO3 Ag2CrO4 + 2KNO3 Ksp[AgCrO4]= 1.7X 10-12
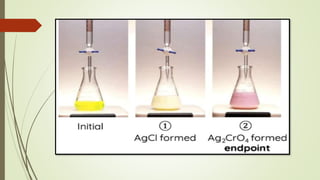
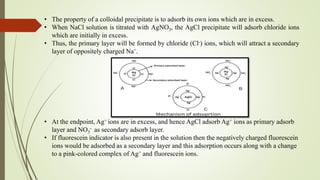
Chlorides and bromides are used as analytes in this method.
The pH range in Mohr’s method is 6.5-7.5](https://image.slidesharecdn.com/precipitationtitrationdpharm-240314151754-01a19df0/85/precipitation-titration-dpharm-1st-year-6-320.jpg)