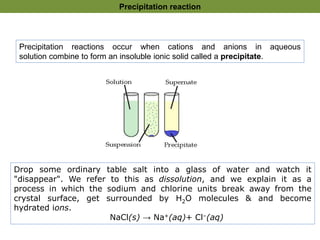
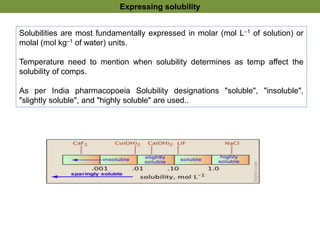
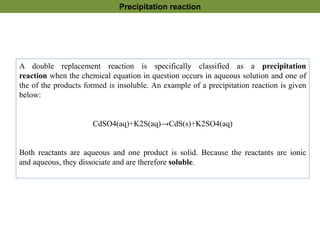
1. Precipitation titrations involve the titration of an analyte solution with a reagent that causes the formation of an insoluble precipitate.
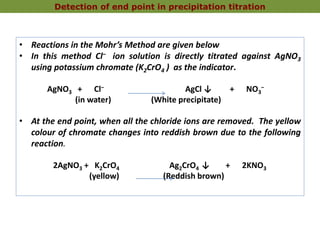
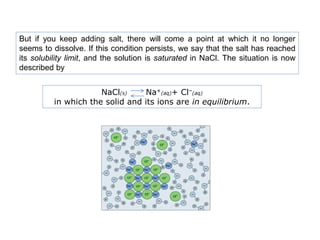
2. The Mohr method is commonly used for chloride determination and involves titrating a chloride solution with silver nitrate using potassium chromate as an indicator.
3. At the endpoint, excess silver nitrate reacts with potassium chromate to form a reddish brown silver chromate precipitate, indicating that all the chloride ions have been precipitated out as silver chloride.

![Solution process and solubility product:
Solubility depend upon the breaking up of solute solute interaction by solute
solvent interactions. When solvent overcome the solute forces of crystal then
crystal get soluble.
Like dissolve like
During precipitation the opposite condition as mentioned above is desired.
Intermolecular forces between molecules are high and solute forces replaces
the solute solvent forces.
Solubility product for the reaction
AB A+ + B-
K sp = [A+ ] + [B-]
Substance will precipitate out when the product of ionic concentration exceeds
the Ksp value i.e. in above equation of Ksp, the BA will precipitate out when the
product of out of [A+ ] [B-] exceed Ksp
Theory of precipitation](https://image.slidesharecdn.com/15387205-230214190901-f498787a/85/Precipitation-Titrations-7-320.jpg)

![4. Calculate conc of each ion:
Each mole of magnesium carbonate dissociate and form 1 gm ion of magnesium
and 1 gm ion of carbonate. Hence both ion have same concentration equal to
molar solubility
[Mg++ ] = [CO3- - ] = 6.32 x10-3
5. Substitute values and calculate Ksp
Ksp = 6.32 x10-3 X 6.32 x10-3
Ksp + 4 X10-6
Calculations of solubility product](https://image.slidesharecdn.com/15387205-230214190901-f498787a/85/Precipitation-Titrations-11-320.jpg)
![Detection of end point in precipitation titration
In this method, first the analyte react with the titrant after the analyte is reacted
completely the next drop if titrant react with indicator and formed small quantity
of colored precipitate which indicate end point of titration (Mohr’s method)
Example:
Assay of NaCl with silver nitrate with dilute potassium chromate solution as
indicator.
Ksp(AgCl) = 1.2 x 10-10 = [Ag+] [Cl-]
Ksp(Ag2CrO4) =1.7 x 10-12 = [Ag+2] [CrO4
-2]
We expect that the salt with smaller Ksp should precipitate first, this is true if both
salt dissociate to yield same number of ions. But in this case the chloride ions
are in excess than that of chromate ion and concentration of chromate ion very
dilute i.e. 0.0014M, hence the chloride precipitate first and then chromate will
precipitate as colured compound](https://image.slidesharecdn.com/15387205-230214190901-f498787a/85/Precipitation-Titrations-13-320.jpg)