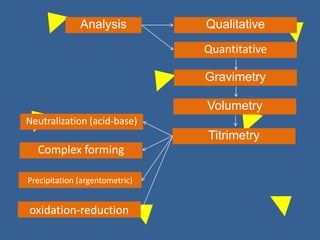
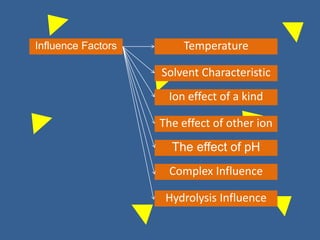
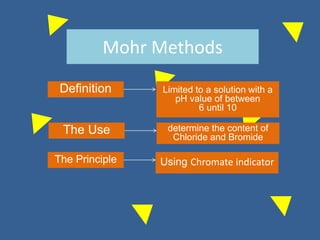
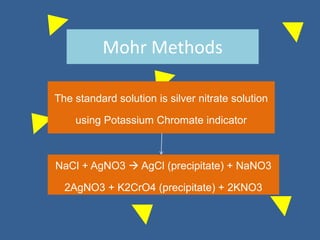
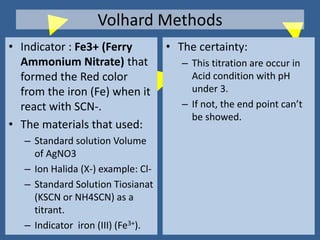
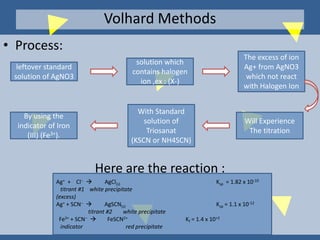
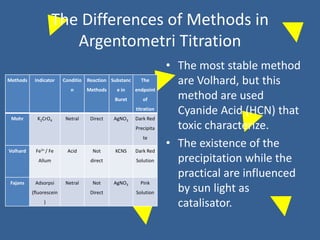
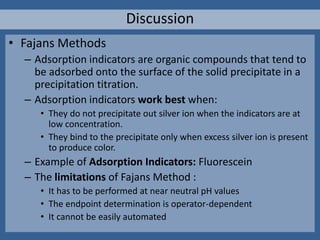
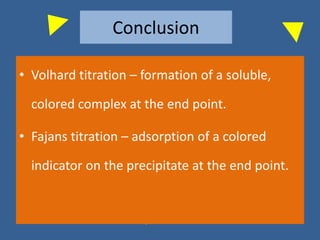
The document outlines various methods of argentometric titration, including Mohr, Volhard, and Fajans methods, focusing on their procedures, indicators, and conditions required for accurate results. It discusses the specific reactions, titrants, and indicators used, as well as limitations and factors affecting the titration processes. Additionally, it highlights equipment and reagents necessary for the experiments and includes references for further reading.