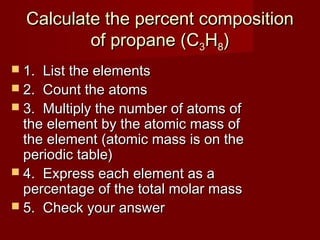
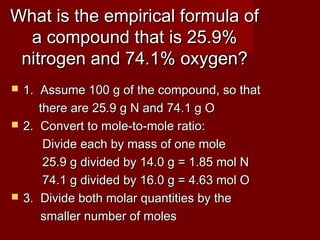
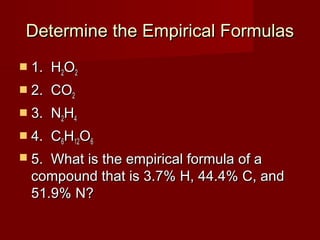
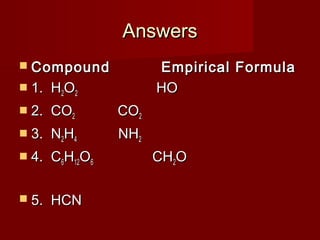
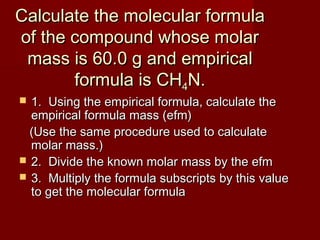
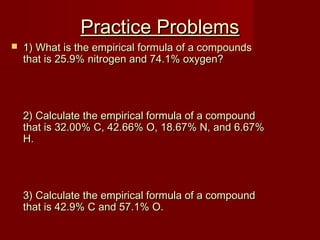
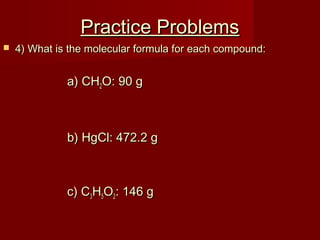
This document provides information and examples for calculating percent composition, empirical formulas, and molecular formulas of compounds. It defines key terms like percent composition and empirical formula. It then works through examples of calculating the percent composition of magnesium and oxygen that form a compound, the percent composition and mass of carbon in propane, and determining empirical formulas from elemental percentages or mole ratios. The document explains how to calculate molecular formulas from empirical formulas and molar masses. Finally, it provides practice problems for the reader to work through.