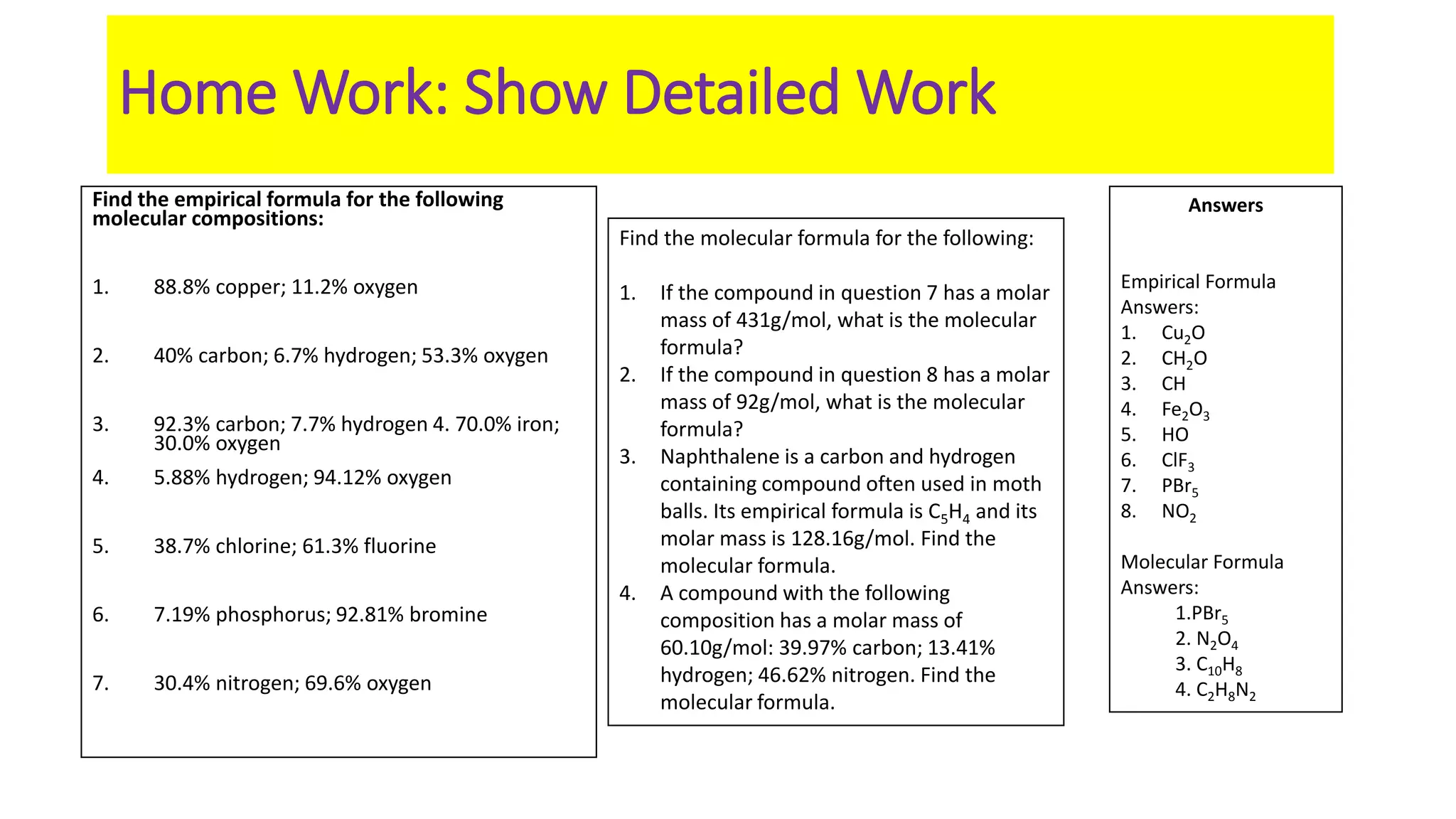
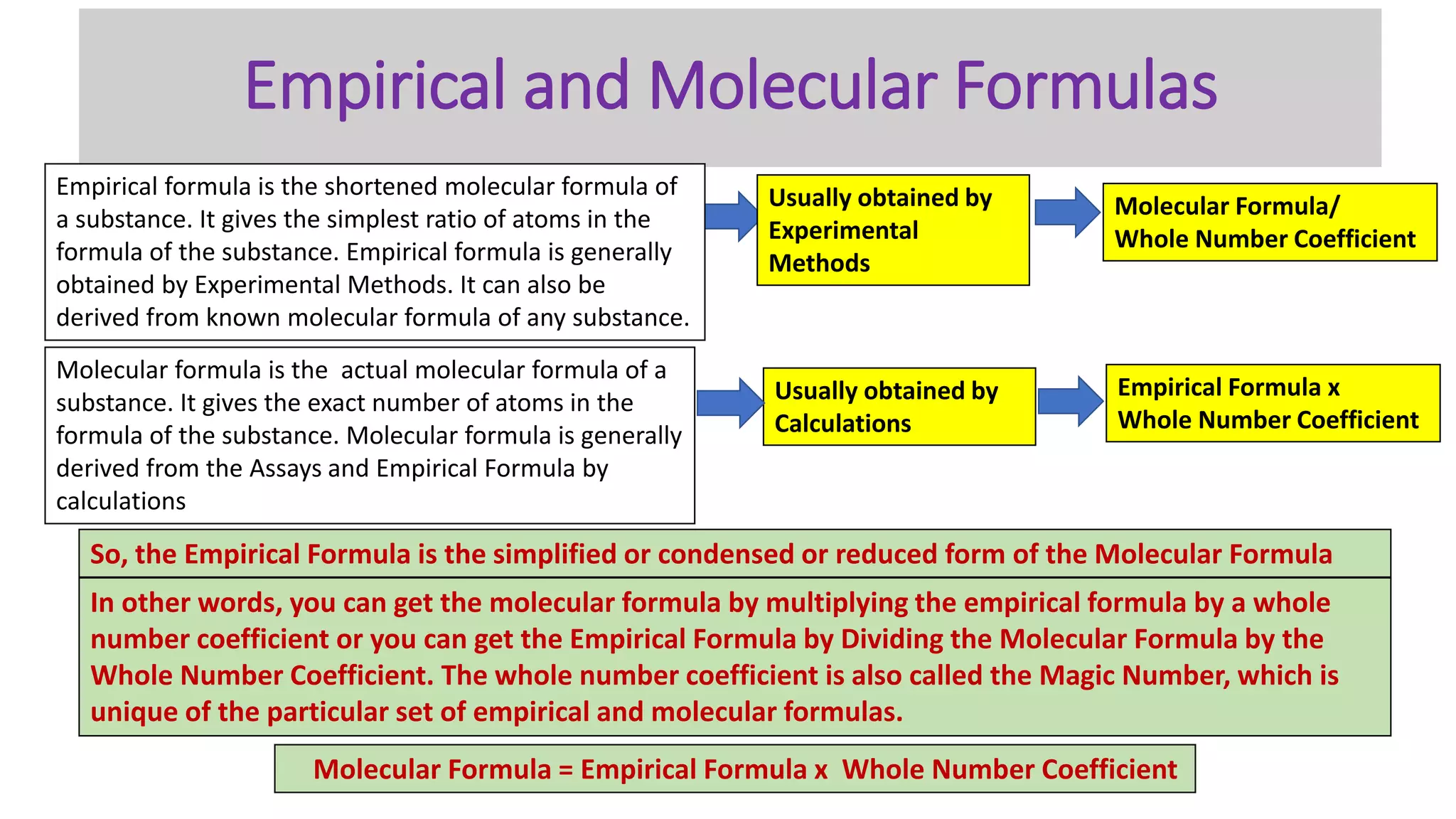
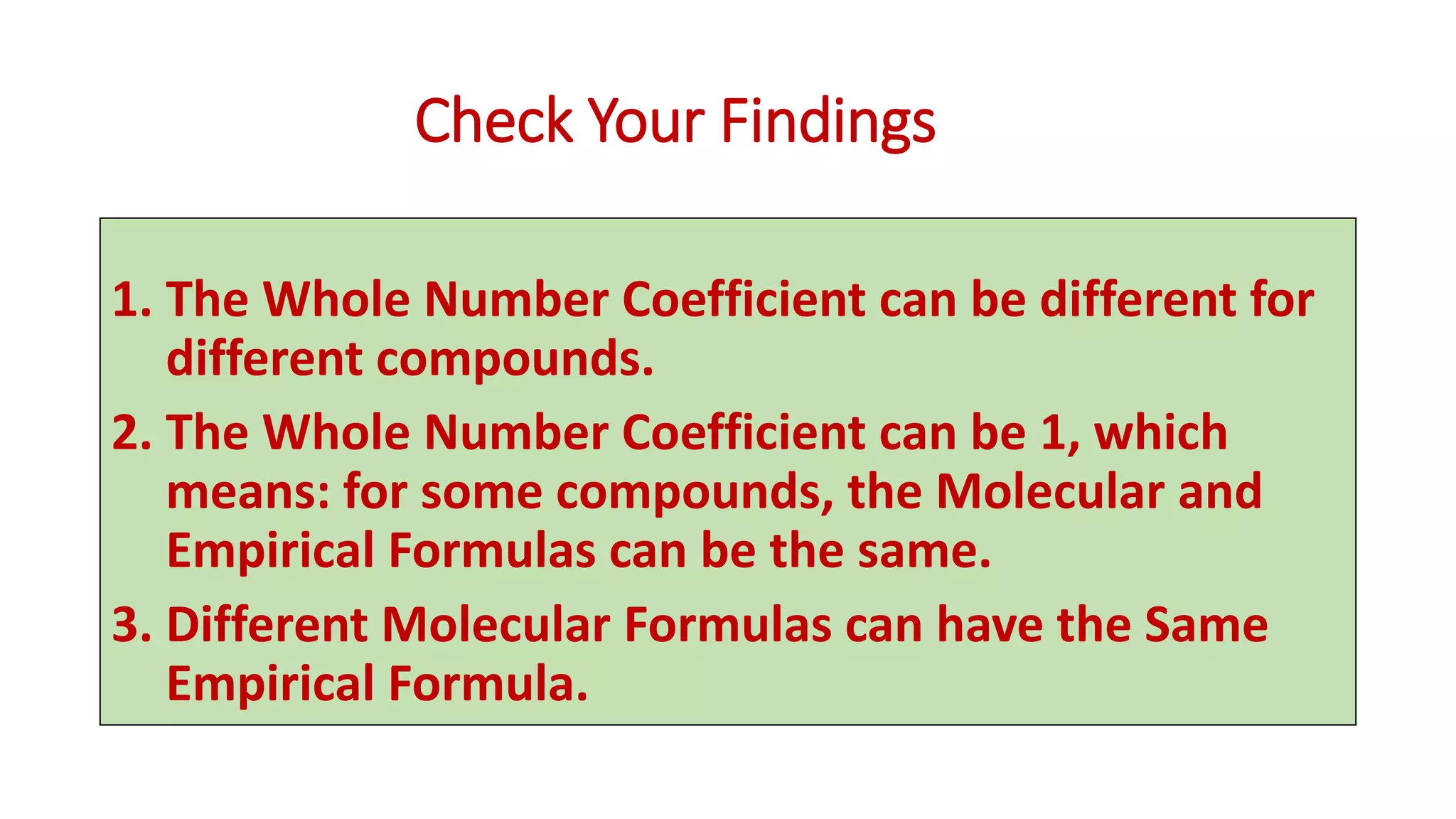
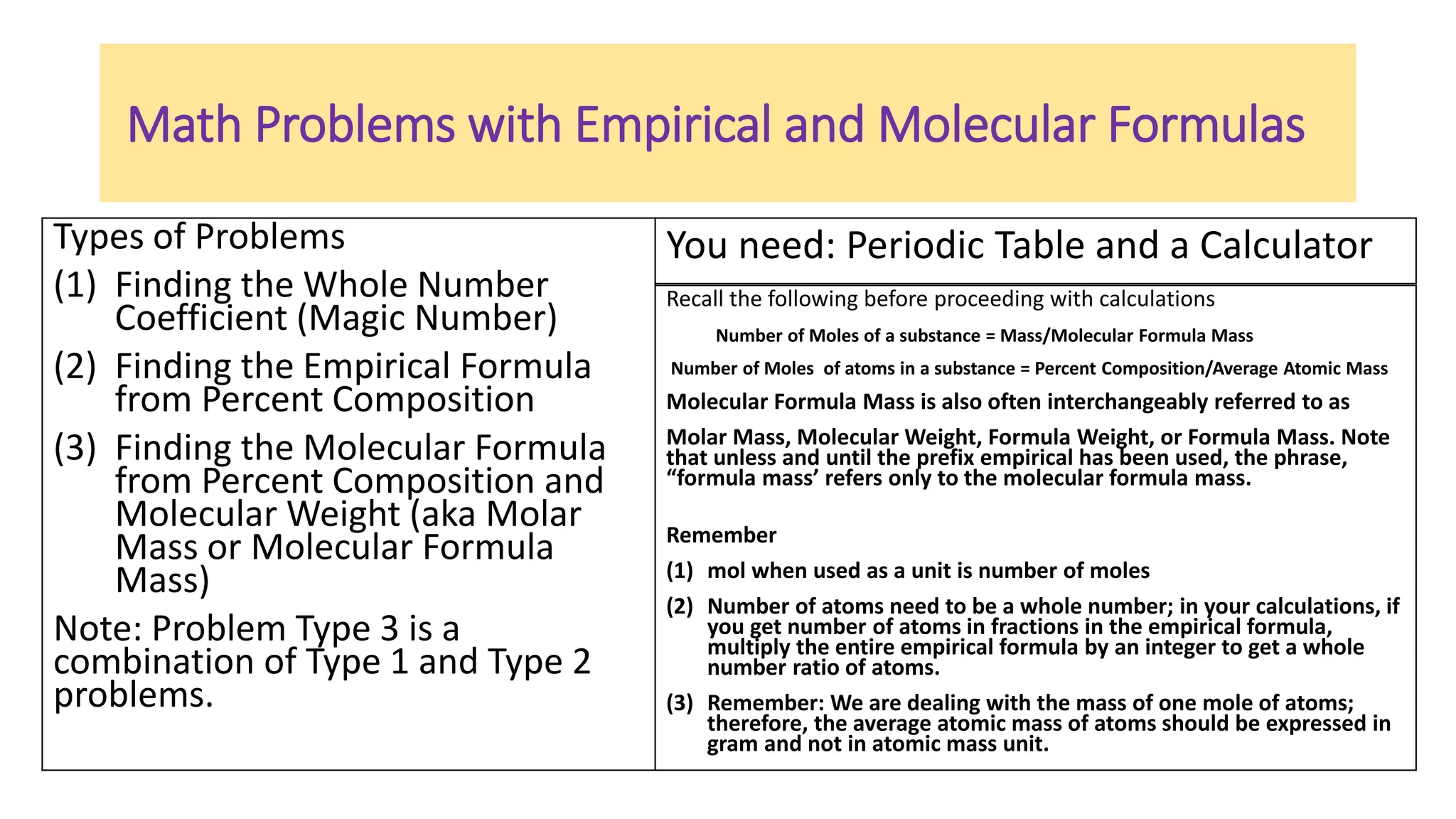
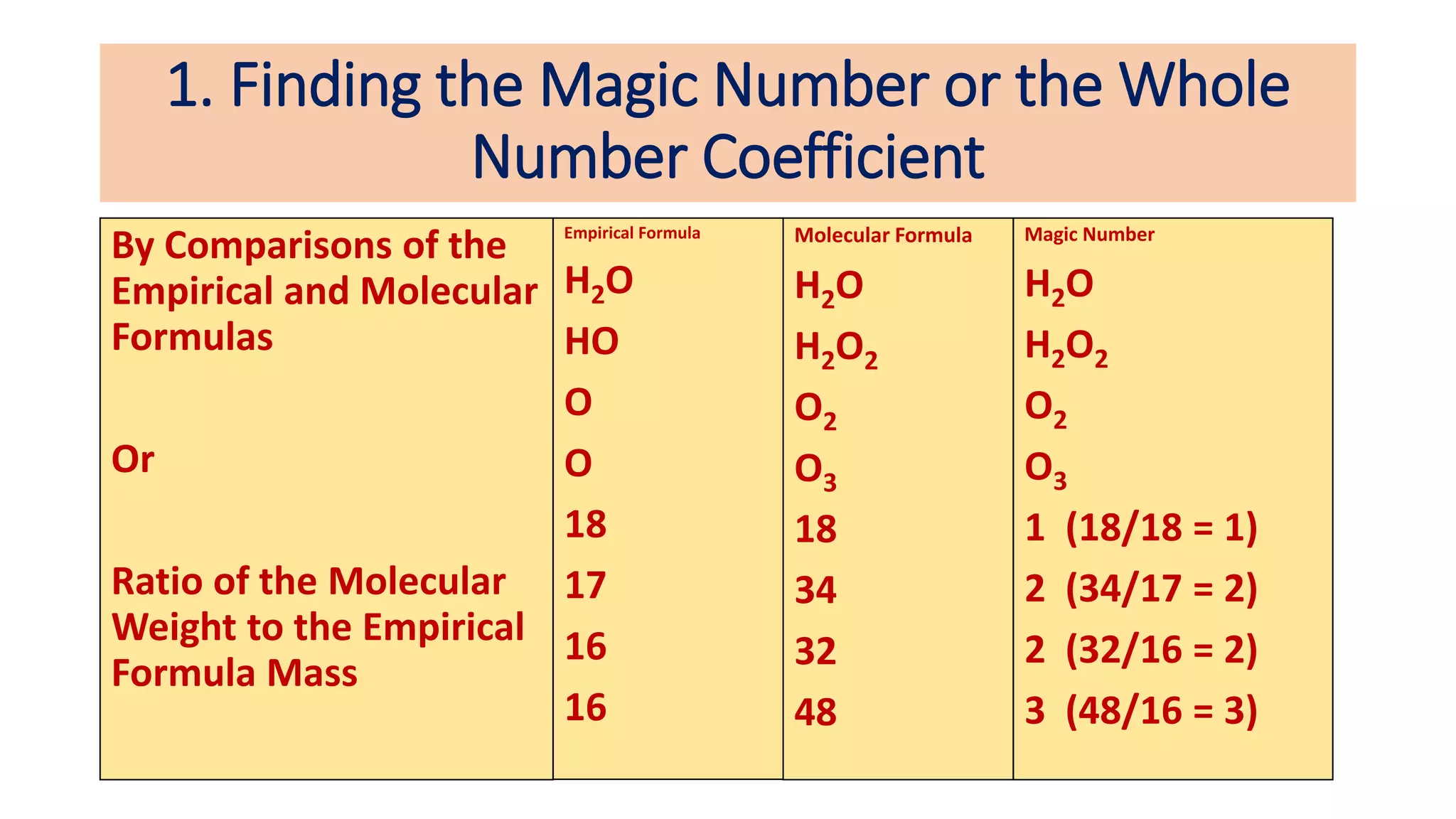
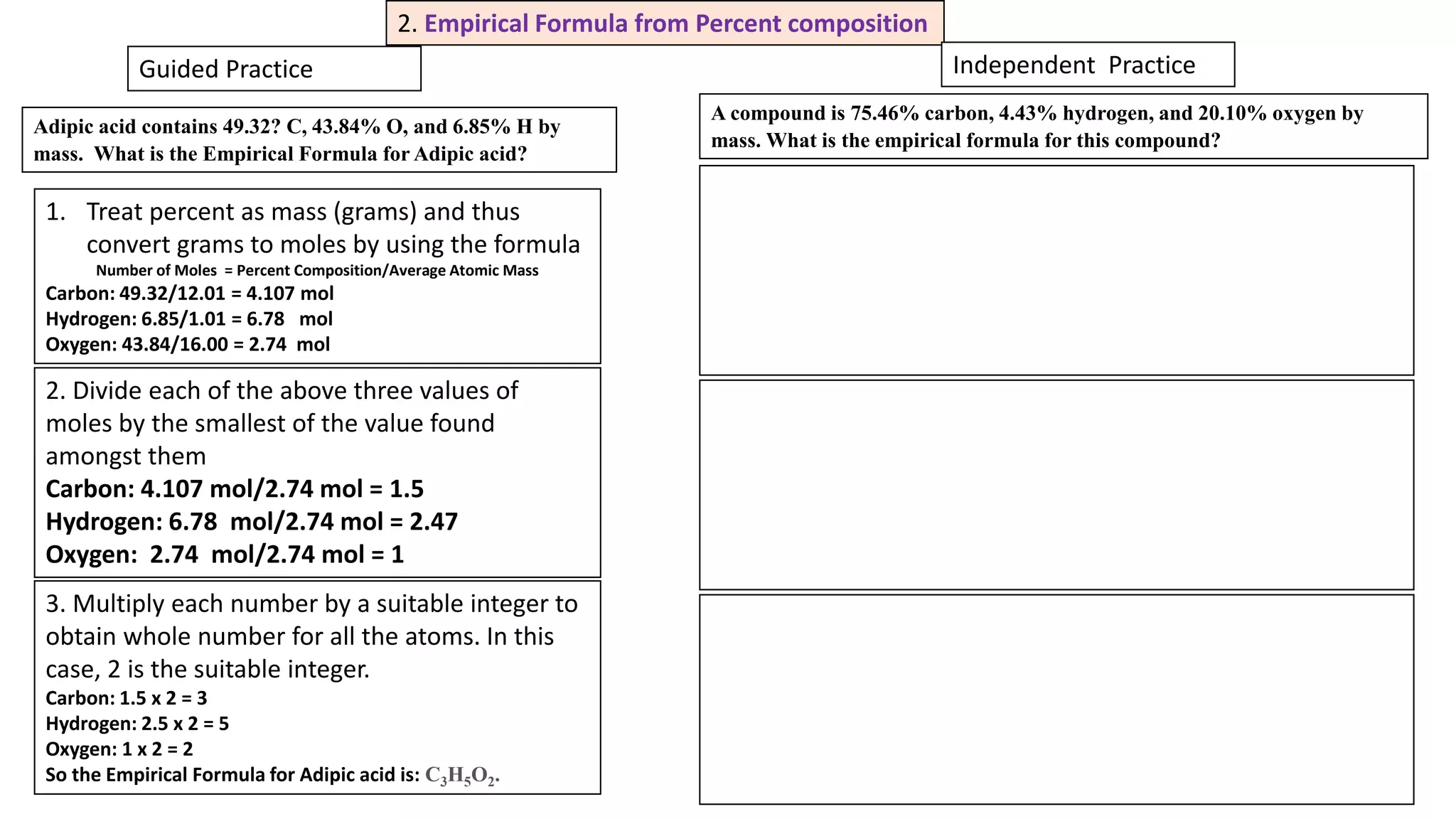
The document explains the concepts and calculations of empirical and molecular formulas in chemistry, detailing how to derive one from the other and their unique coefficients. It provides a variety of practice problems for finding empirical and molecular formulas based on percent composition and molecular weights. Key points include the role of whole number coefficients, different problem types, and the importance of proper calculations using atomic masses.



![3. Molecular Formula from Empirical Formula
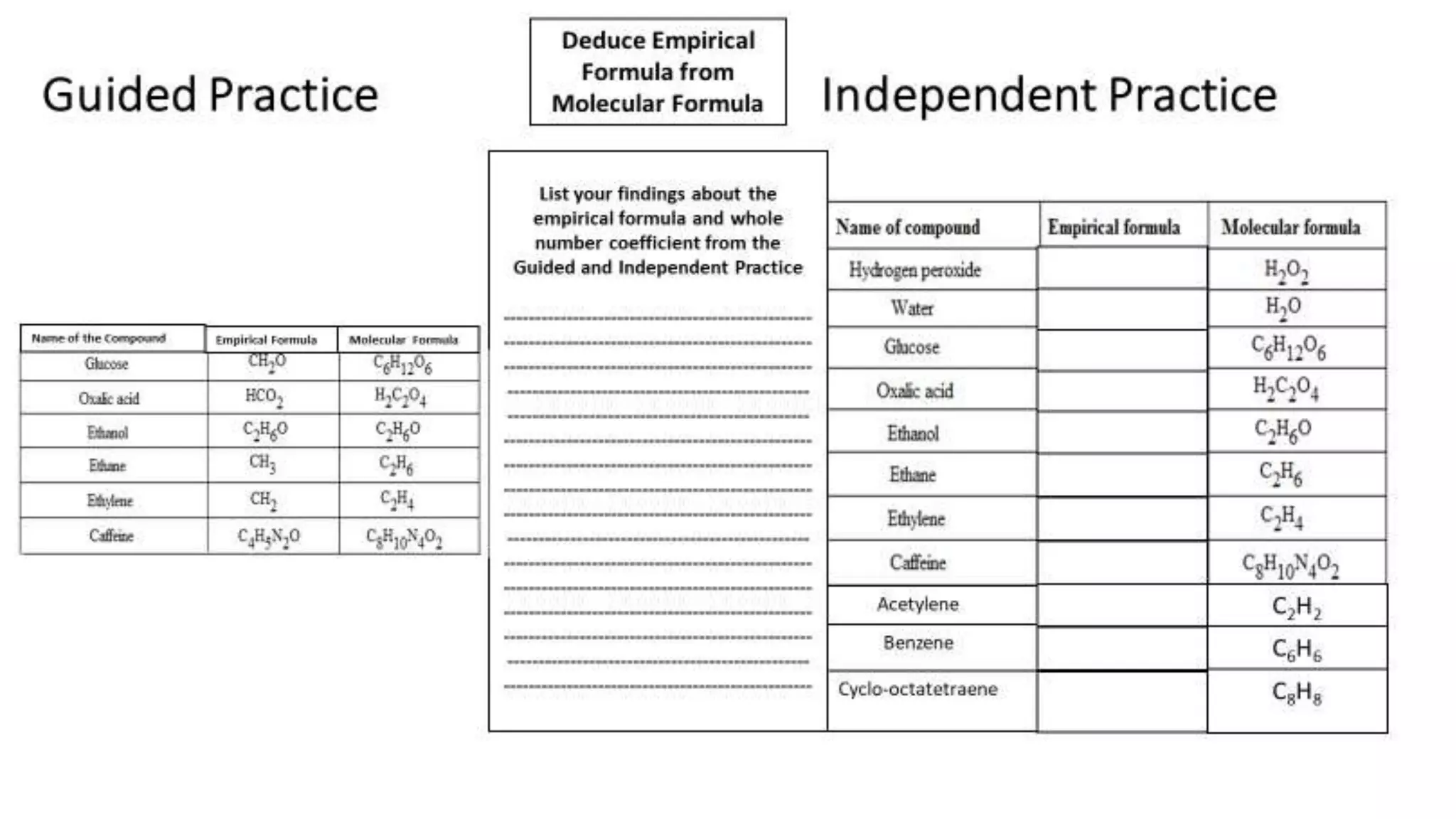
Independent Practice
A compound with an empirical formula of C10H7O2 has a formula mass of
318.31 g/mol, find its molecular formula
Guided Practice
The empirical formula for adipic acid is C3H5O2. The
molecular weight of adipic acid is 146 g/mol. What is the
molecular formula of adipic acid?
1. Find the Mass of the Empirical Formula of C3H5O2
2. Divide the Molecular Weight by the mass of the
Empirical Formula to get the Whole Number Coefficient
3. Multiply the Empirical Formula by the Whole Number
Coefficient
The Molecular Formula for Adipic Acid is C6H10O4
146/73 = 2
3(12.01 g) + 5(1.01 g) + 2(16.00 g) = 73.08 g
[C3H5O2] x 2 = C6H10O4](https://image.slidesharecdn.com/empiricalandmolecularformulas-180102011936/75/Empirical-and-molecular-formulas-11-2048.jpg)