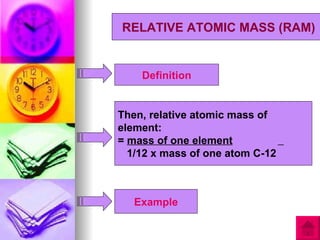
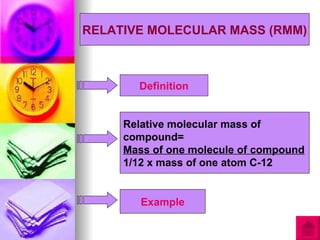
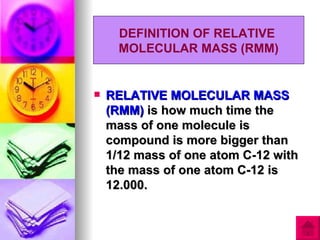
1) Relative atomic mass (RAM) is a measurement of how many times heavier an atom is than 1/12 the mass of one carbon-12 atom. Relative molecular mass (RMM) similarly compares the mass of a molecule to 1/12 the mass of one carbon-12 atom.
2) Scientists first used hydrogen as the standard for RAM/RMM but switched to oxygen and finally settled on carbon-12 due to its stability and prevalence.
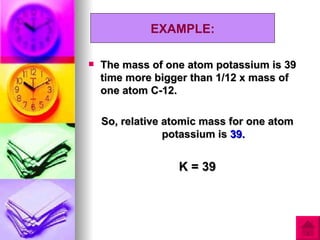
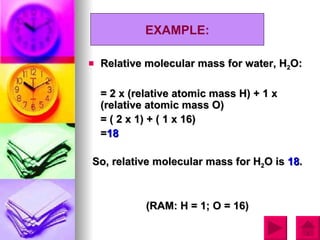
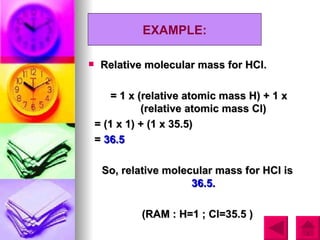
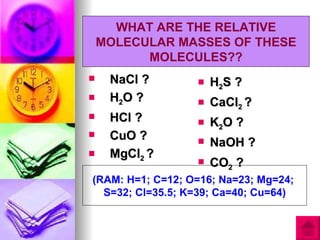
3) The document provides examples of calculating RAM and RMM values for various elements and compounds using the relative scales and given atomic mass values.