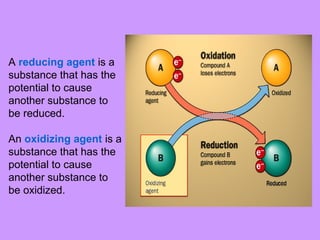
This document discusses redox (reduction-oxidation) reactions. It defines oxidation as the loss of electrons and reduction as the gain of electrons. It explains how to identify oxidizing and reducing agents, assign oxidation numbers, and balance redox reactions using the half-reaction method. Key steps include writing separate half-reactions for oxidation and reduction and adjusting coefficients to balance the number of electrons lost and gained. Disproportionation reactions are also introduced, where a substance acts as both an oxidizing and reducing agent.