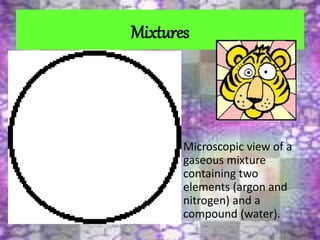
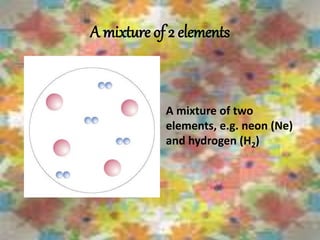
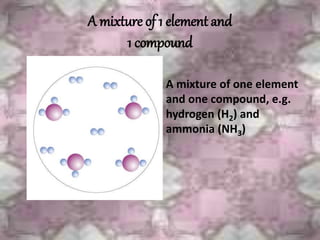
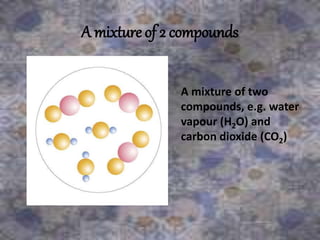
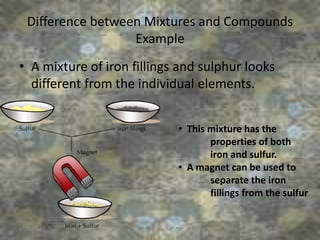
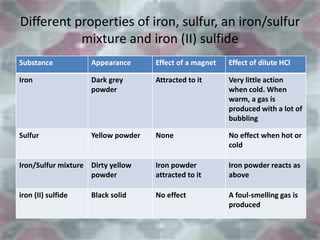
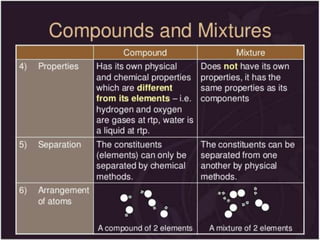
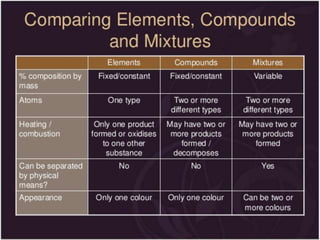
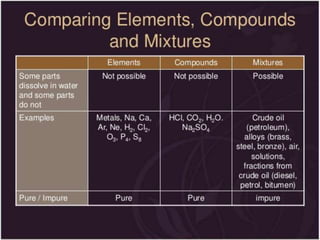
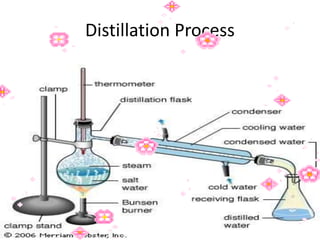
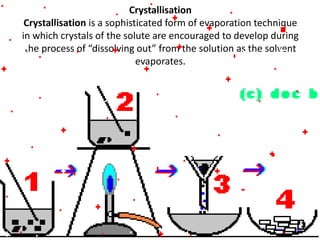
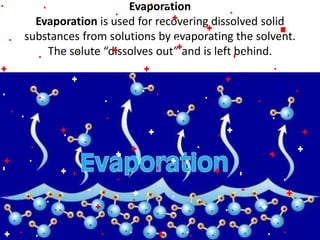
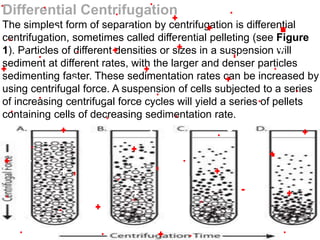
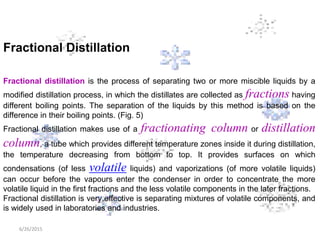
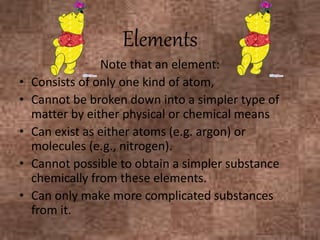
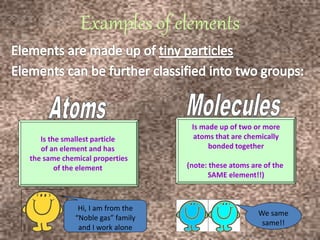
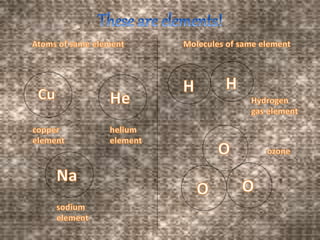
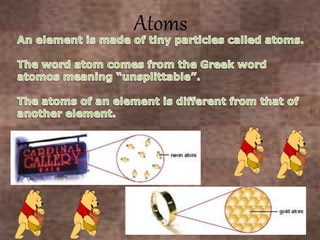
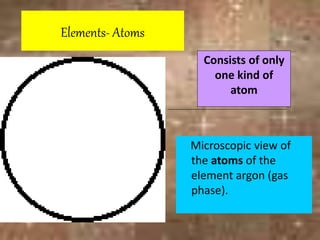
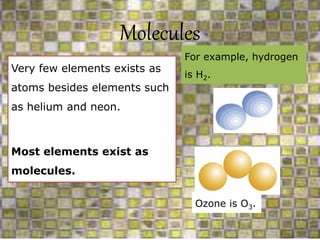
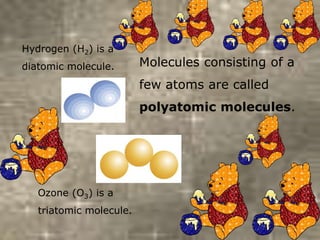
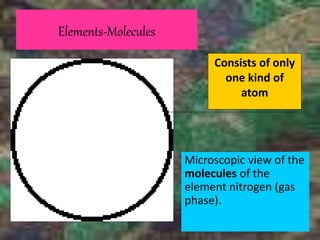
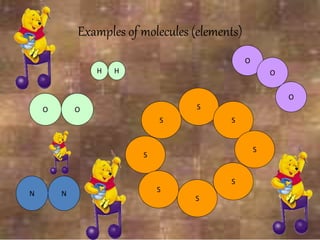
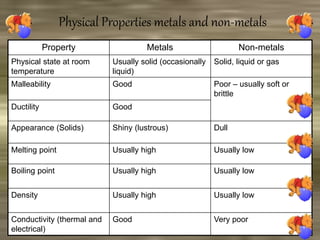
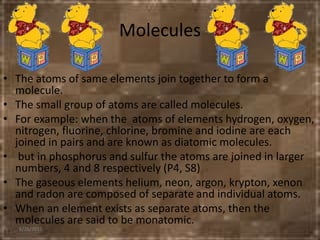
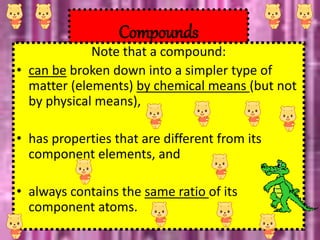
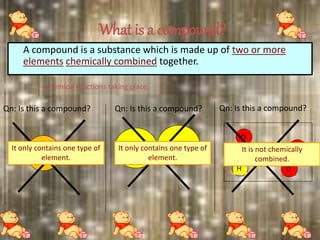
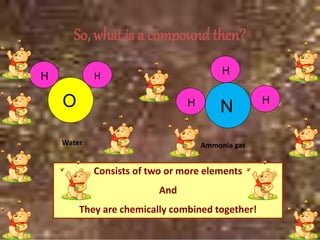
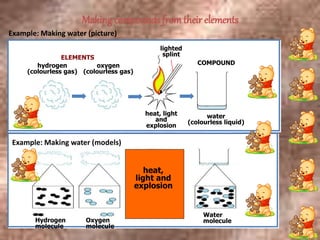
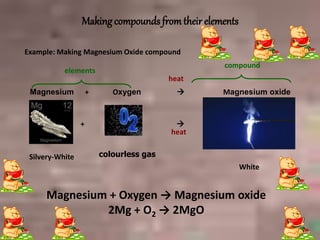
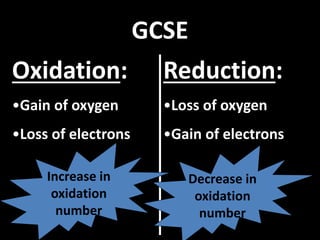
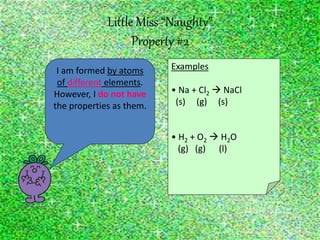
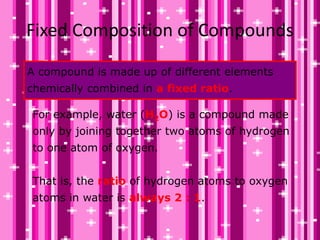
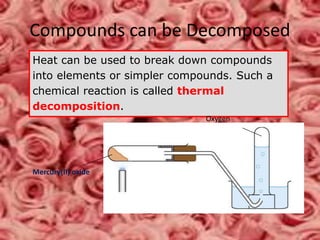
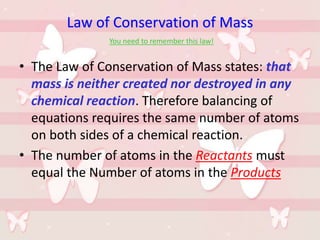
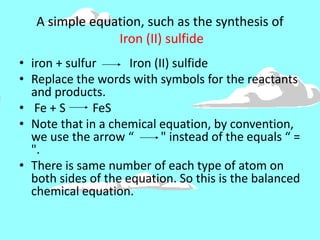
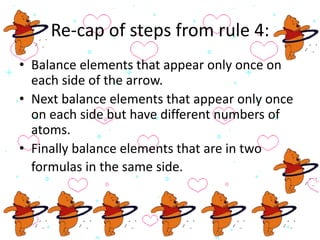
This document discusses the classification and properties of matter. It defines the four states of matter as solid, liquid, gas, and plasma. Matter is classified as either elements, compounds, or mixtures based on its chemical constitution. Elements are pure substances that cannot be broken down further, while compounds contain two or more elements chemically bonded together. Compounds have distinct properties from their constituent elements. The document provides examples of elements and compounds, and discusses their distinguishing physical and chemical properties. Redox reactions are described as reactions where both oxidation and reduction occur simultaneously.





































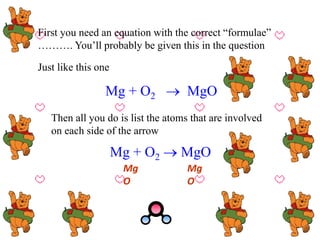
![[1] Just count up the atoms on each side
Then start balancing:
Mg + O2 MgO
Mg
O
1
1
1
2
[2] The numbers aren’t balanced so then add “BIG”
numbers to make up for any shortages
And adjust totals
Mg + O2 MgO
Mg
O
1
1
1
2
2
2
2](https://image.slidesharecdn.com/elementscompoundandmixture-150626154408-lva1-app6892/85/Elements-compound-and-mixture-69-320.jpg)

![Try to balance these equations using the same
method:
[1] Na + Cl2 NaCl
[2] CH4 + O2 CO2 + H2O
[4] Al + O2 Al2O3
[3] Li + HNO3 LiNO3 + H2](https://image.slidesharecdn.com/elementscompoundandmixture-150626154408-lva1-app6892/85/Elements-compound-and-mixture-71-320.jpg)
![How did you get on??
[1] 2 Na + Cl2 2 NaCl
[2] CH4 + 2 O2 CO2 + 2 H2O
[4] 4 Al + 3 O2 2 Al2O3
[3] 2 Li + 2 HNO3 2 LiNO3 + H2
Here are the answers:](https://image.slidesharecdn.com/elementscompoundandmixture-150626154408-lva1-app6892/85/Elements-compound-and-mixture-72-320.jpg)