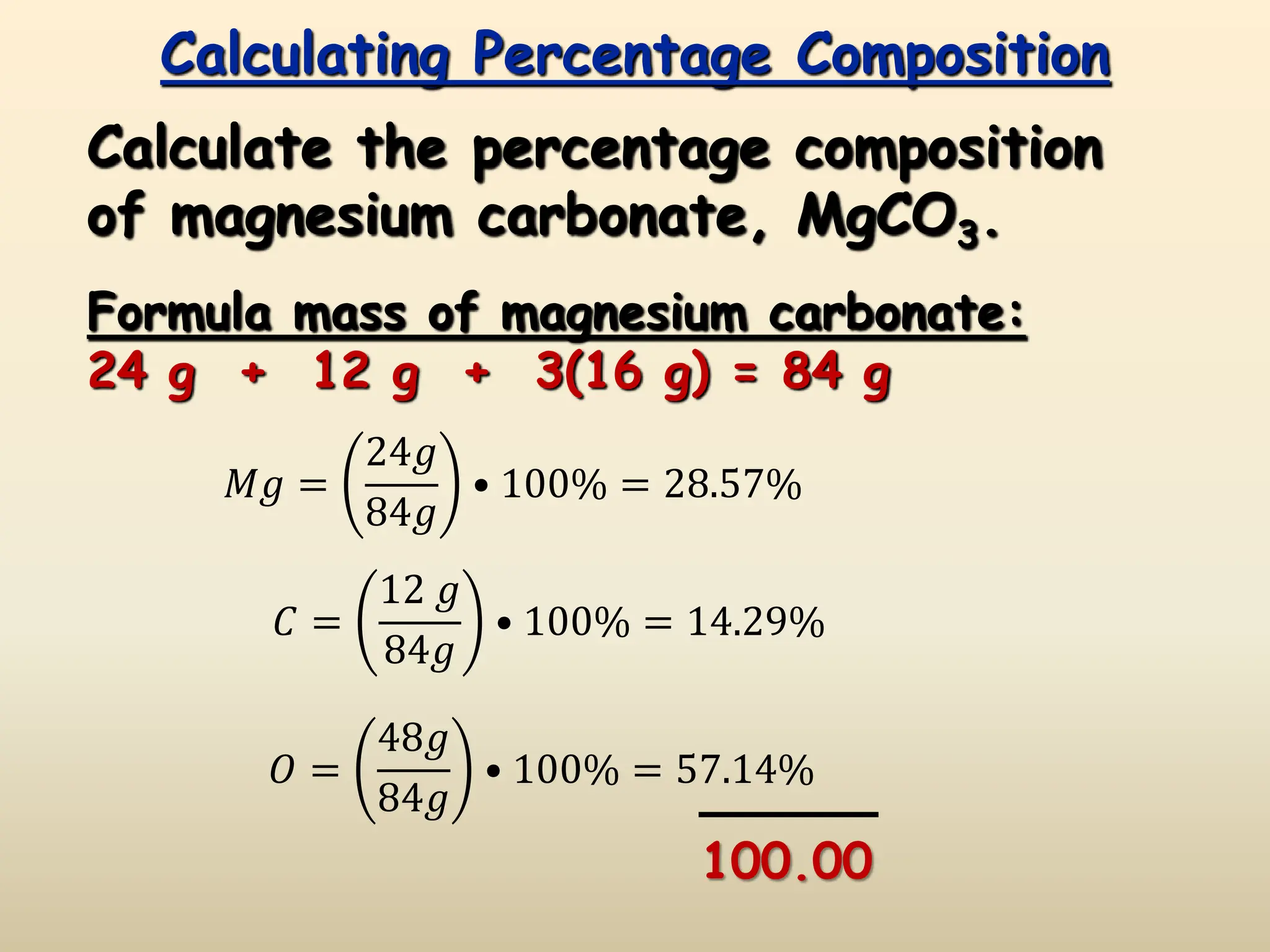
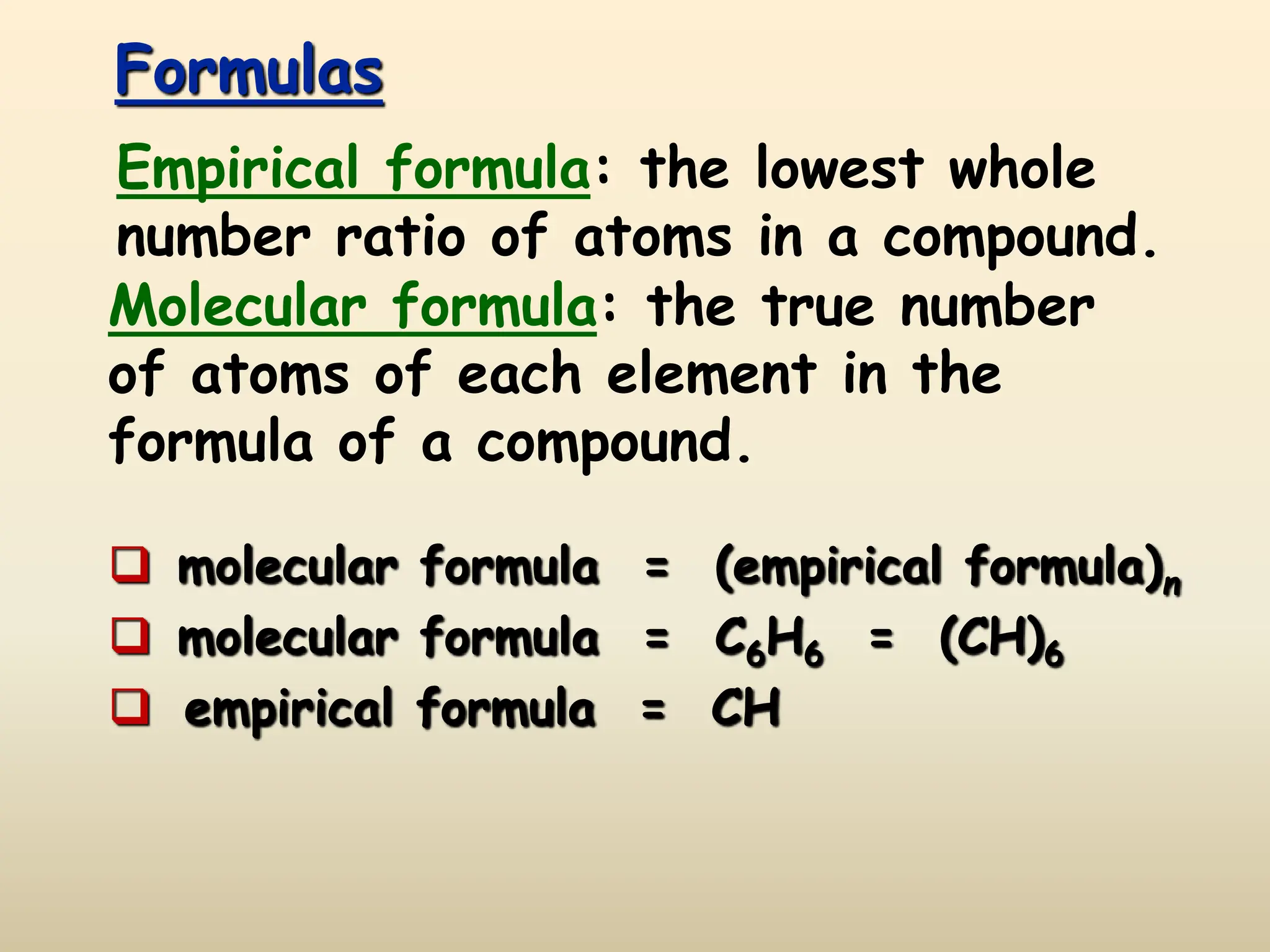
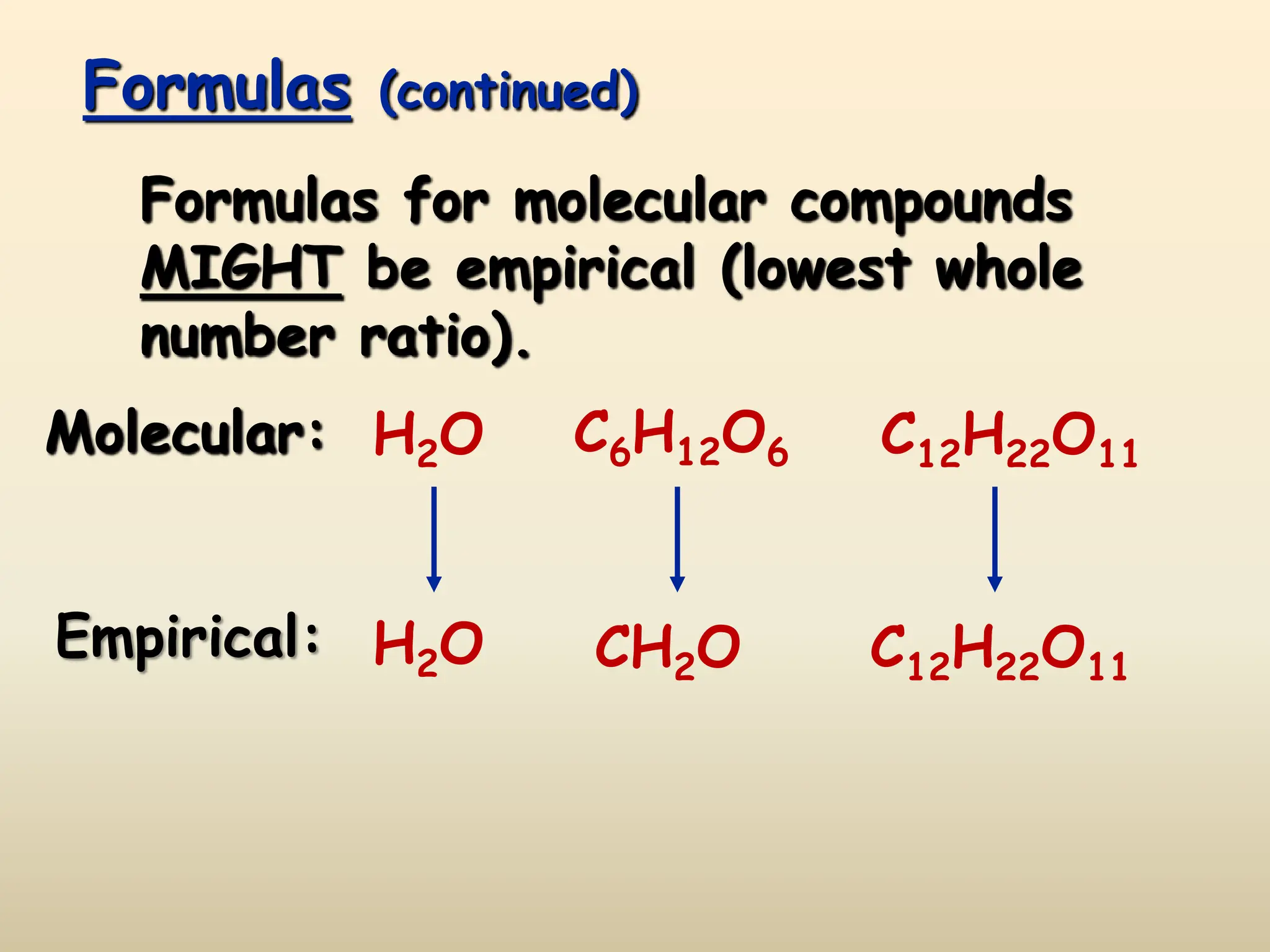
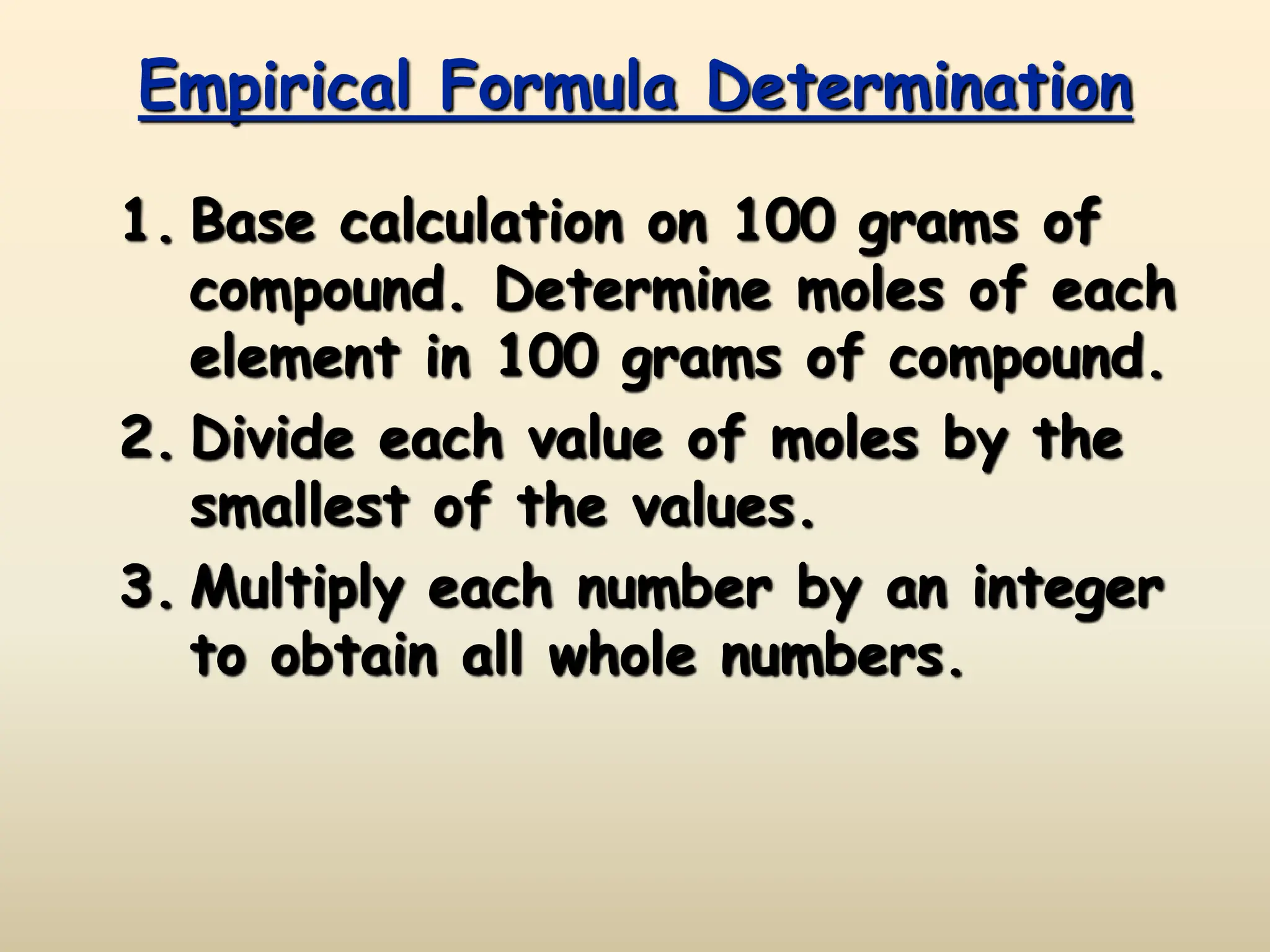
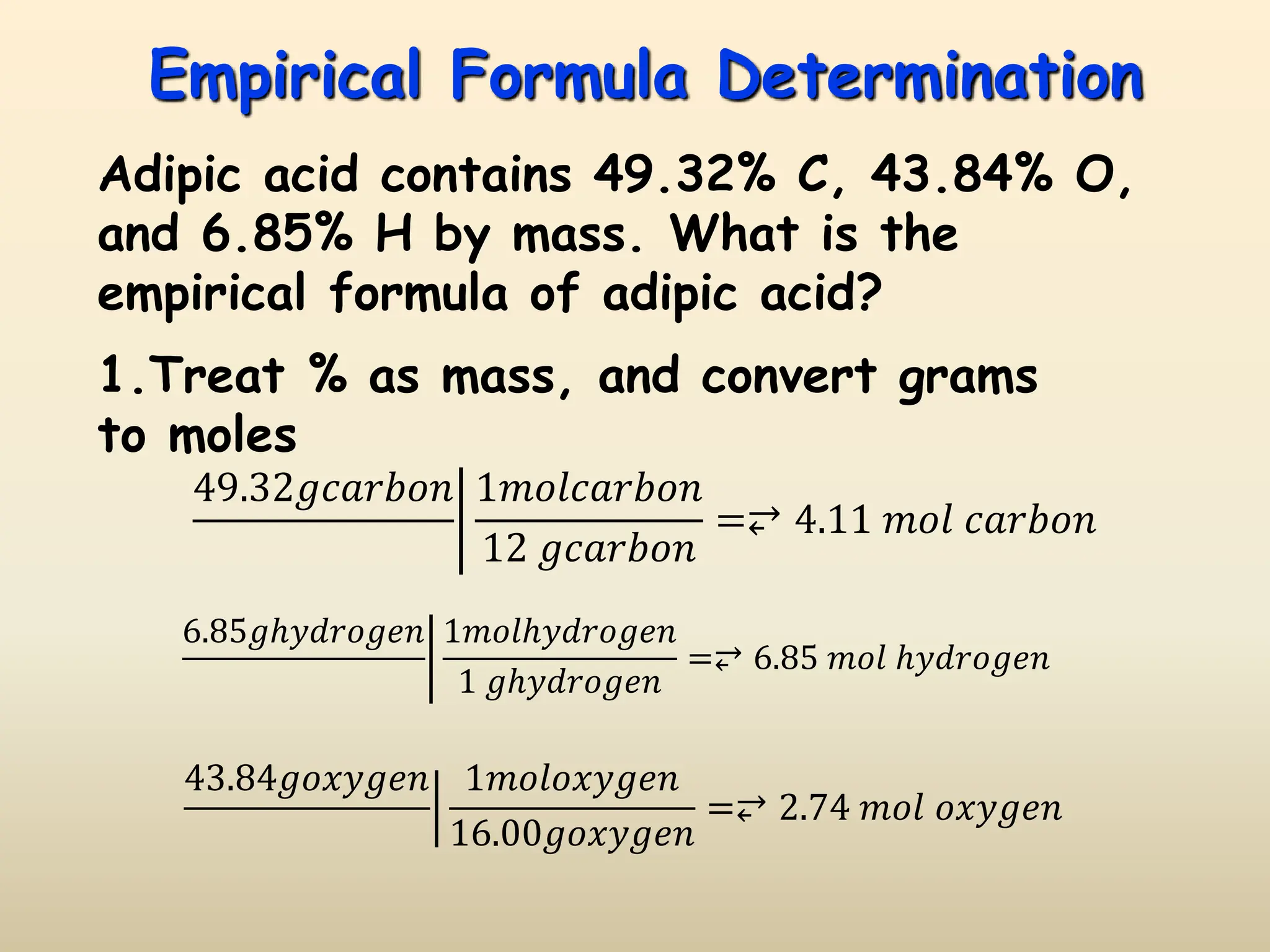
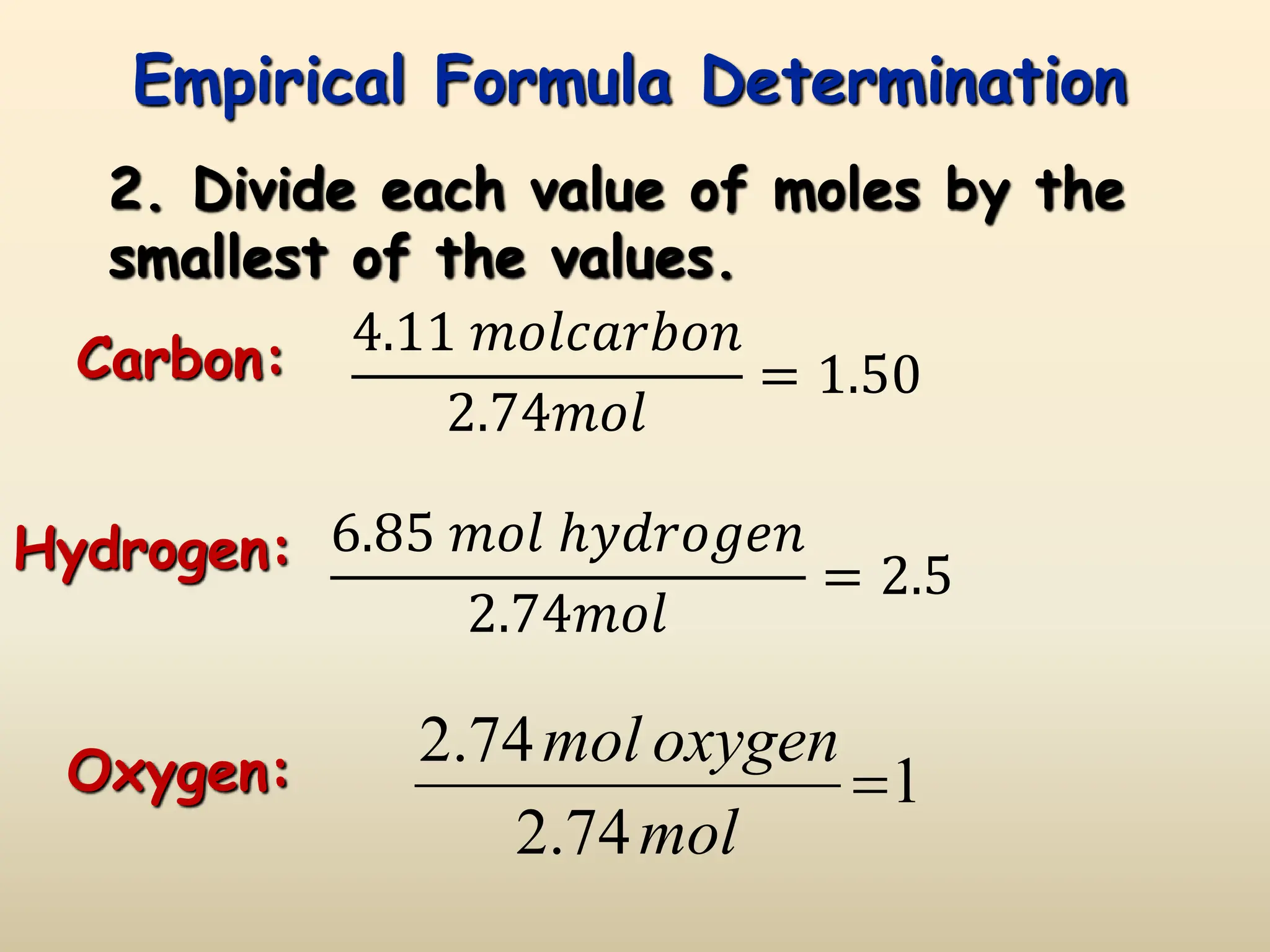
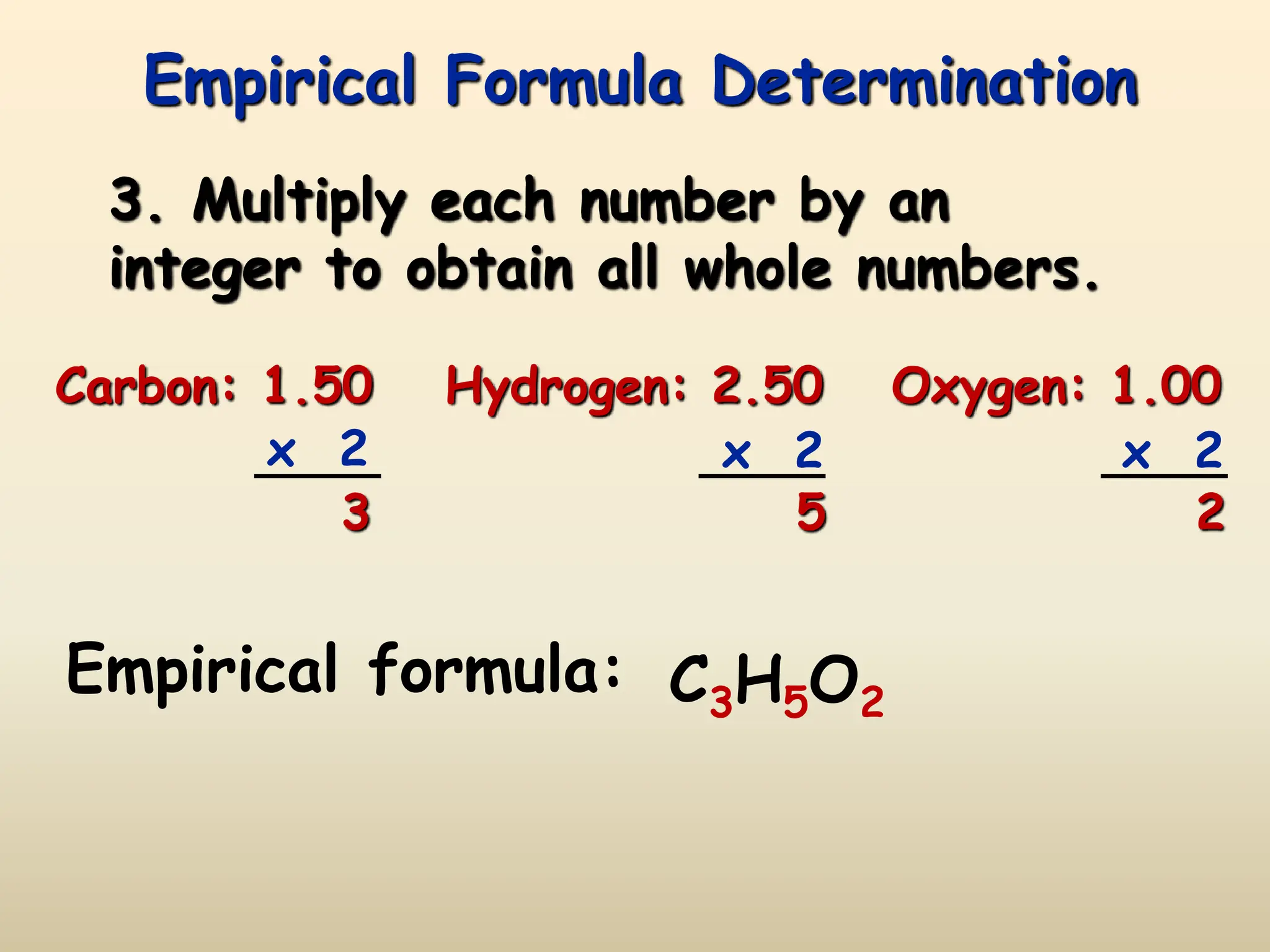
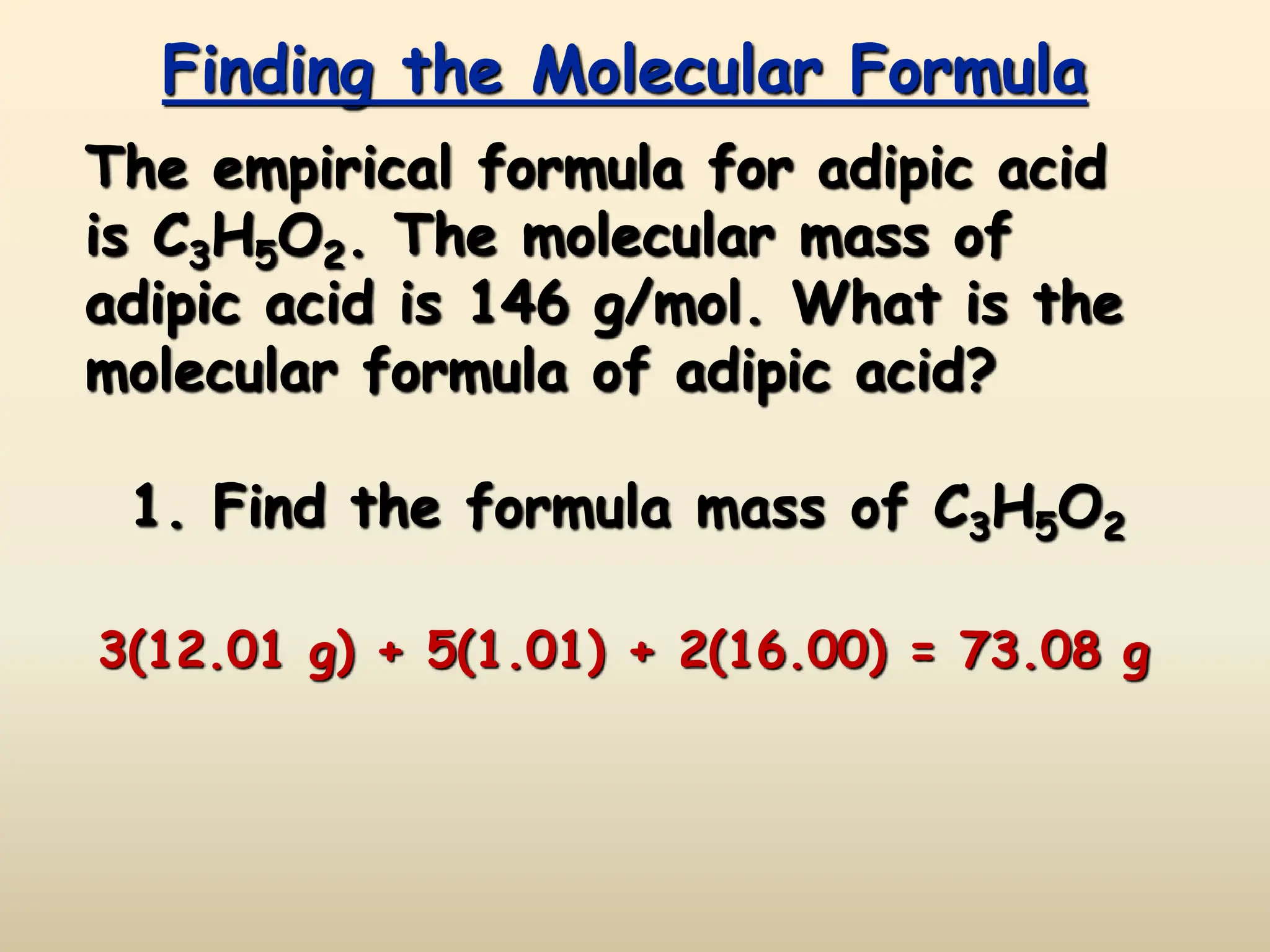
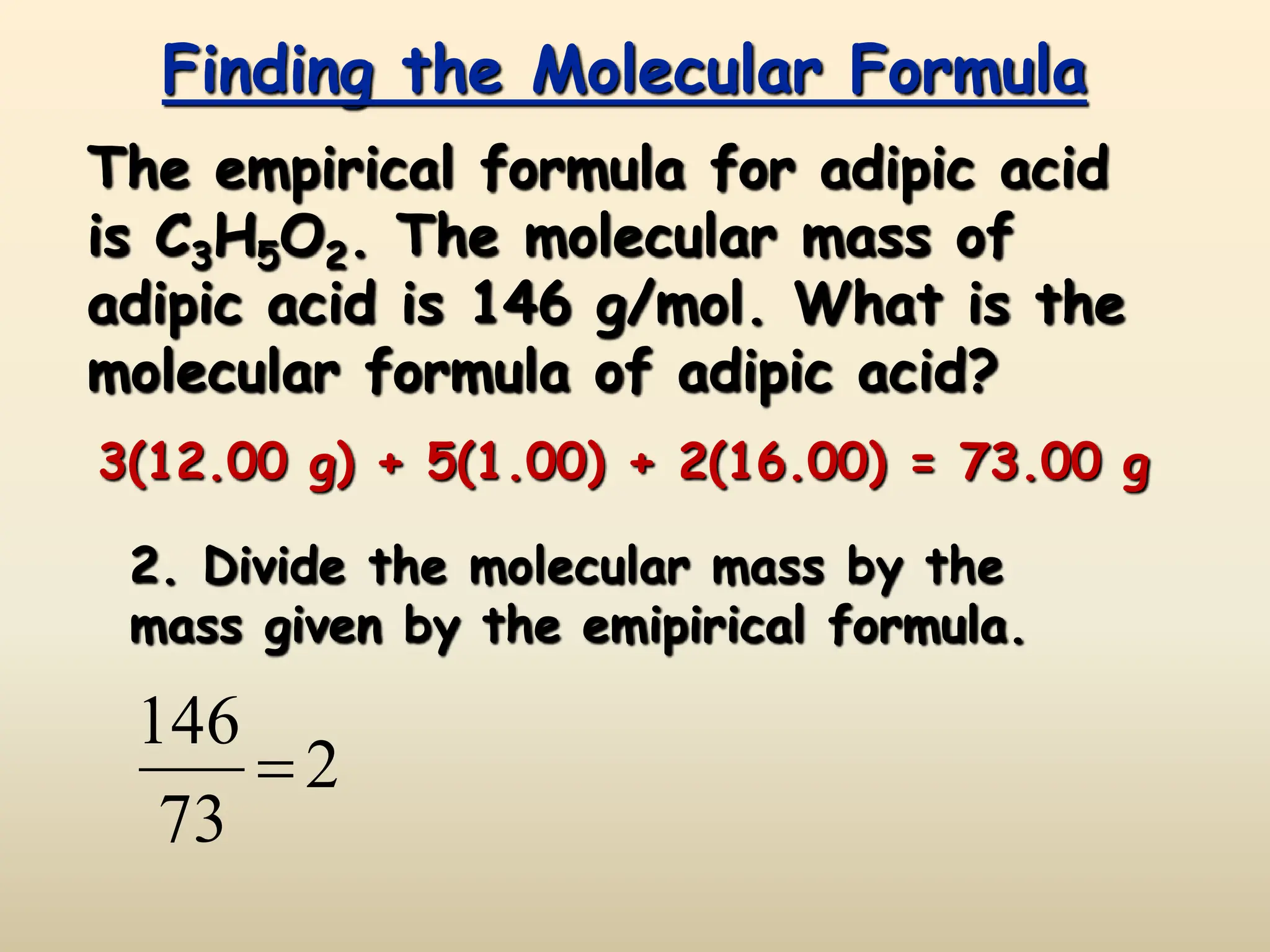
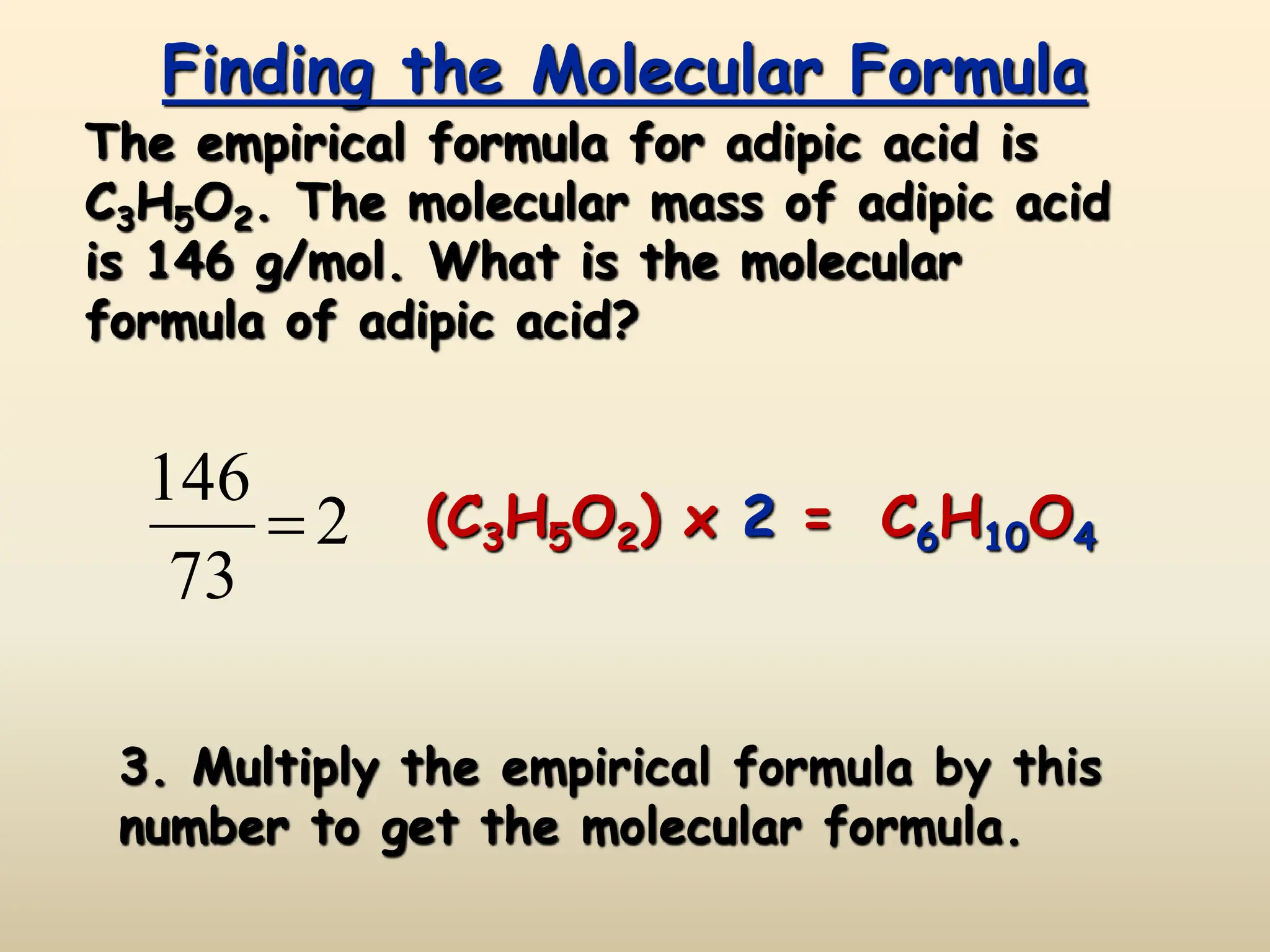
This document discusses calculating percent composition, empirical formulas, and molecular formulas. It provides examples of determining the percent composition of magnesium carbonate and the empirical formula of adipic acid. The key differences between empirical and molecular formulas are also explained: empirical formulas show the lowest whole number ratio of atoms in a compound, while molecular formulas show the actual number of each type of atom. Formulas for ionic compounds are always empirical, but formulas for molecular compounds may be either empirical or molecular.