The document discusses stoichiometry and includes the following key points:
- Homework assignments are listed and students are asked to see the teacher about presentations.
- Stoichiometry is like following a recipe, with balanced equations read in terms of moles.
- Word problems on specific pages are to be examined after working through an example together.
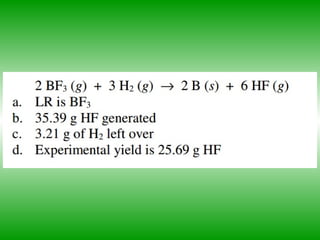
- Limiting reagents are reactants that are used up first and limit the amount of products that can be produced. Excess reagents are not completely used up.
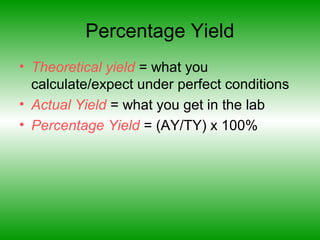
- Theoretical yield is calculated while actual and percentage yields refer to results obtained in the lab.