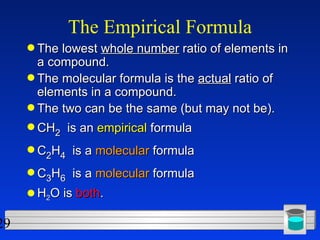
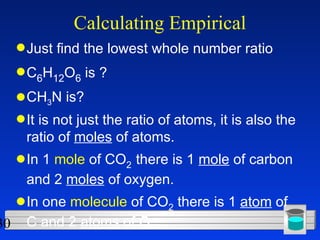
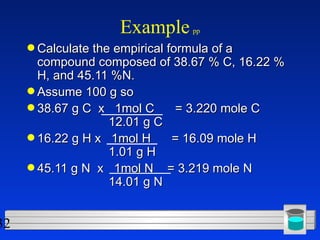
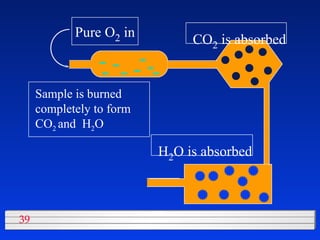
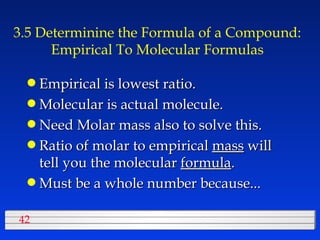
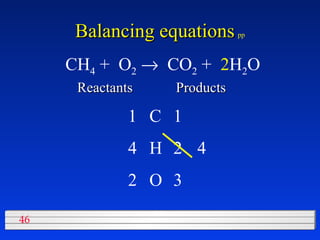
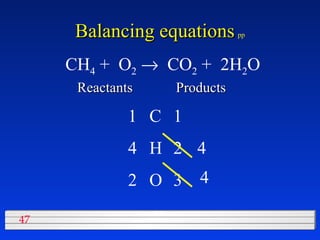
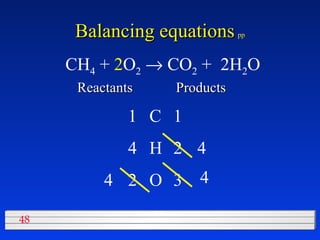
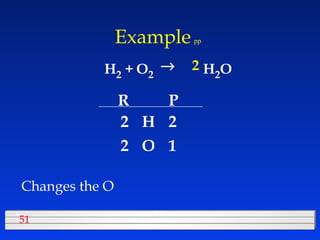
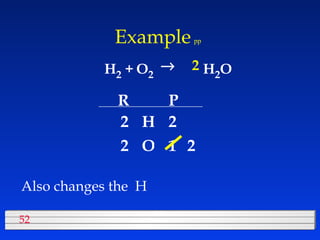
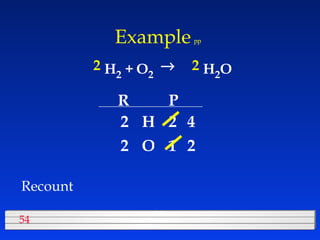
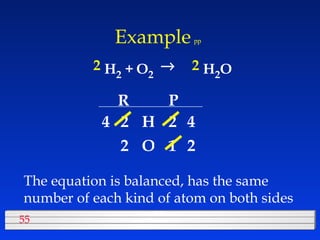
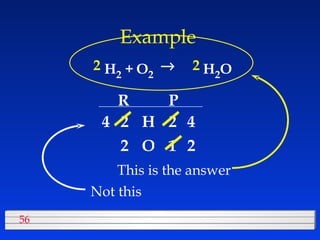
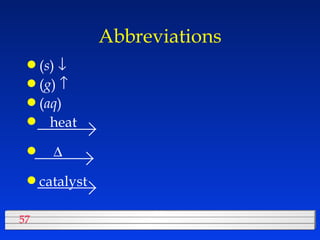
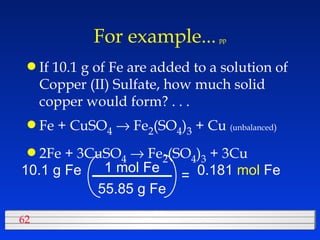
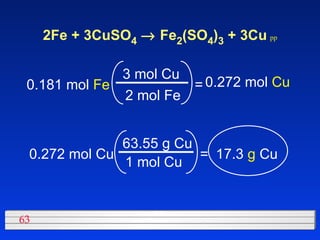
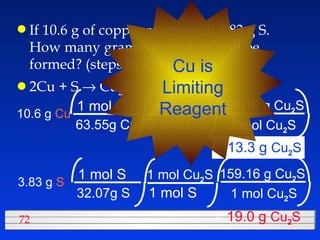
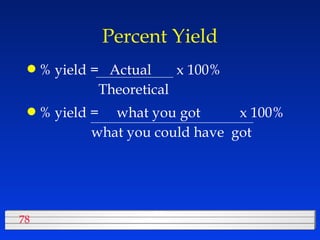
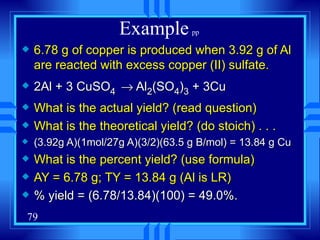
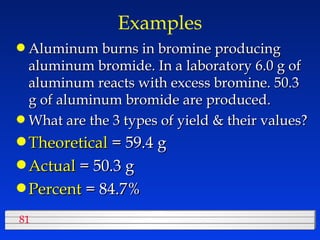
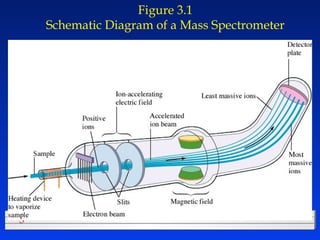
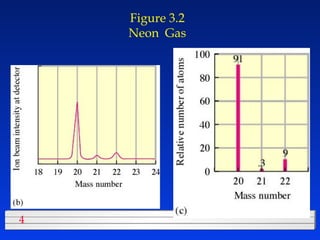
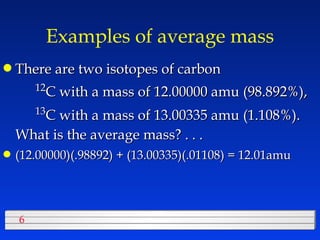
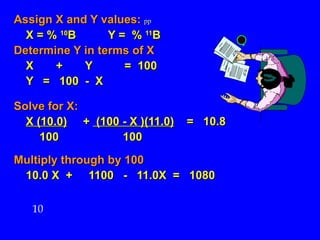
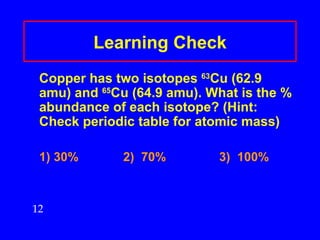
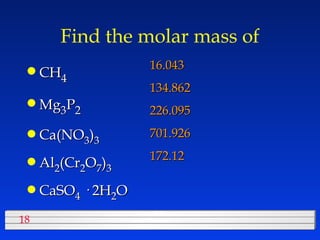
This document discusses atomic mass and isotopes. It begins by explaining that an atomic mass unit (amu) is used to discuss the mass of atoms, where 1 amu is 1/12 the mass of a carbon-12 atom. Atomic masses listed in the periodic table are in amu. Isotopes have different atomic masses that result in the average atomic mass not being a whole number. Examples are provided to demonstrate calculating average atomic masses from the masses and abundances of isotopes.















![Example pp Calculate the percent composition of a compound that is composed only of 29.0 g of Ag and 4.30 g of S. The answer is . . . 87.1% Ag from [29.0 ÷ (29.0 + 4.30)] x 100%. What is the % of S? . . . 12.9% (must add up to 100%).](https://image.slidesharecdn.com/ch3z53stoich-110115225255-phpapp02/85/Ch3-z53-stoich-23-320.jpg)