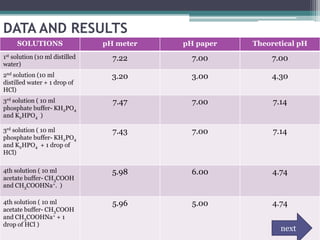
The document discusses pH, buffers, and buffer solutions. It begins by introducing pH and defining buffers. It then outlines the objectives, methodology, data and results of an experiment preparing phosphate and acetate buffer solutions. The results showed the buffers maintained pH when HCl was added. The discussion explains how buffers work to resist pH changes. It concludes that buffers are effective at maintaining pH within their given ranges, and understanding buffers and equations is crucial for preparation and analysis.


![INTRODUCTION
• pH- introduced in 1909 • Buffer- it is an aqueous
by Sorensen. solution consisting of a
- it is defined as mixture of a weak acid and
its conjugate base or a weak
negative log of Hydrogen
base and its conjugate acid. It
ion concentration. can resists pH change.
• There are solutions in Basically, it is use in keeping
calculating the pH. Here the pH at a nearly constant
are some of them: value in a wide variety of
a. Calculation of [H-] chemical applications. In
b. Calculating the base 10 biochemistry, one good
example of buffer solution
log of H-
found in nature is blood
c. pH is negative of the which is present in all
value found in base 10 living organisms.
log](https://image.slidesharecdn.com/phandbuffer-130221002404-phpapp01/85/P-h-and-buffer-3-320.jpg)

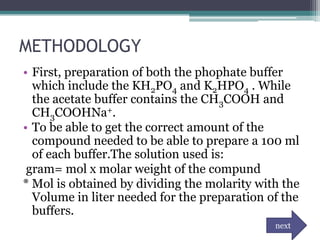
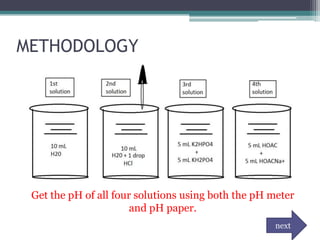
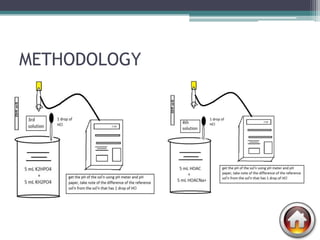
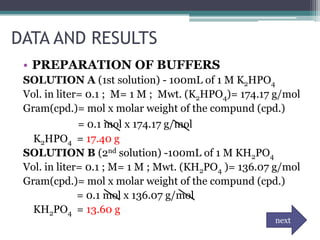

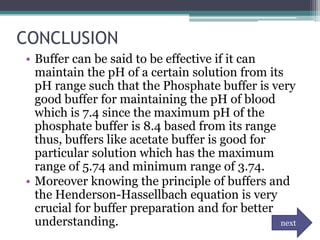
![DATA AND RESULTS
• To get the pH of water:
Kw= [H+] [OH-]
pH = - log [ 1E-7]
= 7.00
• To get the pH of water after adding 1 drop of HCl.
H2O + HCl --------> H3O + Cl
1 drop = 0.00005L
[H+]= 1E-7 + 0.00005
pH = -log [ 0.00005]
=4.30
• To get the pH of the phosphate buffer- 0.005 L K2HPO4 + 00.005 KH2PO4
Dilution formula: (M1V1)= (M2V2)
(1mol)(0.005ml)=(?)(0.01ml)
?mol= 0.005ml/ 0.01ml
=0.5M of K2HPO4 and KH2PO4
• pH= pka + log [salt]/ [acid]
=7.2E-8 + log [0.5]/[0.5]
= 7.14
• To get the pH of the acetate buffer- 0.005 L CH3COOH + 0.005 L CH3COOHNa+
Dilution formula: (M1V1)= (M2V2)
(1mol)(0.005ml)=(?)(0.01ml)
?mol= 0.005ml/ 0.01ml
=0.5M of K2HPO4 and KH2PO4
• pH= pka + log [salt] / [acid]
=1.8E-5 + log [0.5]/[0.5]
=4.74](https://image.slidesharecdn.com/phandbuffer-130221002404-phpapp01/85/P-h-and-buffer-11-320.jpg)



![2. Derive Henderson- Hesselbach Equation
▫Ka = [H=][A-]
[HA]
▫-log Ka= -log = [H=][A-]
[HA]
-log Ka= - log [H] –log [A-]/[HA]
pKa= pH –log [A]/[HA]
pH= pka + log [A-]/ [HA]
next](https://image.slidesharecdn.com/phandbuffer-130221002404-phpapp01/85/P-h-and-buffer-16-320.jpg)
![3. An acetate buffer was prepared by mixing 10 mL of 0.1M acetic acid
and 100 mL of 0.1M sodium acetate. What is the pH of the buffer
solution.
Dilution formula: (M1V1) = (M2V2)
(0.1M acetic acid)(0.01mL)=(?)(0.11mL)
?= 0.001/0.11
M=0.009 CH3COOH
(0.1M sodium acetate)(0.1mL)=(?)(0.11mL)
?= 0.01/0.11
M=0.09 CH3COOHNa+
pH = pka + log [salt]/[acid]
=4.74 + log [0.09]/[0.009]
=5.74
4. Can a buffer solution be prepared from a mixture of NaNo3 and
HNO3? Explain.
*Technically, from the meaning of buffer it must a combination of
weak base/acid and its salt, the combination of NaNo3 and HNO3 is
a strong acid and salt mixture thus it will not form a buffer solution.](https://image.slidesharecdn.com/phandbuffer-130221002404-phpapp01/85/P-h-and-buffer-17-320.jpg)