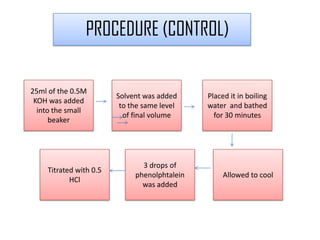
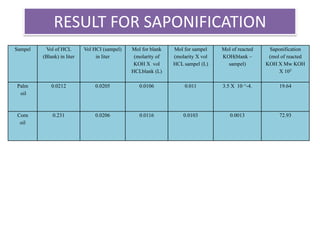
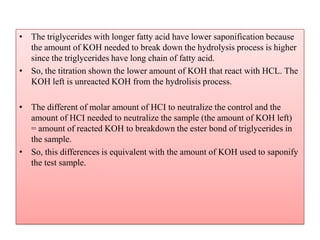
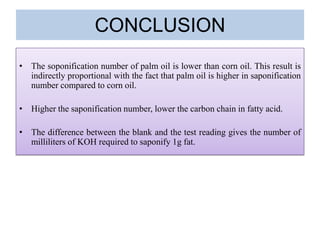
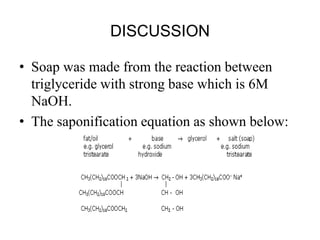
The document summarizes an experiment on determining the saponification number of corn and palm oil. The experiment involves hydrolyzing the oils with potassium hydroxide (KOH) and titrating the leftover KOH with hydrochloric acid (HCl). The saponification number, which indicates the chain length of fatty acids in the oils, is higher for corn oil, meaning it has shorter fatty acid chains than palm oil. However, the results of this experiment showed the opposite trend, likely due to errors in the experimental procedure.